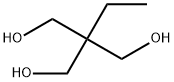
Tris(hydroxymethyl)aminomethane
Synonym(s):Tris;Tromethamine;Tris base;Trometamol;Tris(Hydroxymethyl)aminomethane
- CAS NO.:77-86-1
- Empirical Formula: C4H11NO3
- Molecular Weight: 121.14
- MDL number: MFCD00004679
- EINECS: 201-064-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:09

What is Tris(hydroxymethyl)aminomethane?
Chemical properties
White, crystalline solid. PH (0.1M aqueous solution) 10.6, corrosive to copper, brass, aluminum. Solubility in water 80 g/100 cc (20C). Combustible.
Originator
Trisaminol,Bellon,France,1964
The Uses of Tris(hydroxymethyl)aminomethane
In the synthesis of surface-active agents, vulcanization accelerators, pharmaceuticals. As emulsifying agent for cosmetic creams and lotions, mineral oil and paraffin wax emulsions, leather dressings, textile specialties, polishes, cleaning Compounds, so-called soluble oils. Absorbent for acidic gases. Biological buffer.
The Uses of Tris(hydroxymethyl)aminomethane
tromethamine can help mask odor in a formulation. It is also used as a buffer, keeping product pH stable.
The Uses of Tris(hydroxymethyl)aminomethane
Tris base has been used:
- In the preparation of TAE buffer for TAE agarose gel used to run PCR products by gel electrophoresis
- As a component of sample buffer for SDS-PAGE
- In the preparation of IEC (ion-exchange chromatography) buffer
Background
An organic amine proton acceptor. It is used in the synthesis of surface-active agents and pharmaceuticals; as an emulsifying agent for cosmetic creams and lotions, mineral oil and paraffin wax emulsions, as a biological buffer, and used as an alkalizer. (From Merck, 11th ed; Martindale, The Extra Pharmacopoeia, 30th ed, p1424)
Indications
For the prevention and correction of metabolic acidosis.
What are the applications of Application
Tris Base solution is a high-quality 1.5 M buffer solution containing 0.05% sodium azide as a preservative
What are the applications of Application
Tris base is widely used as a biological buffer or as a component of buffer formulations. Tris base has buffering capabilities in the pH range of 7.0-9.0.
What are the applications of Application
Tris(hydroxymethyl)aminomethane is mainly required for preparation of buffers at physiological range of 7.3 to 7.5. The prepared buffers are compatible with biological fluids. It is of importance to laboratories as a standard pH solution.
Tris(hydroxymethyl)aminomethane has been used as a buffer solution for lactate dehydrogenase assay, in situ hybridization procedure and protein extraction from cells.
Tris(hydroxymethyl)aminomethane is used as a buffer in biochemistry and molecular biology laboratories. It is used as a primary standard to standardize acid solutions for chemical analysis. It finds application in cell membranes to increase permeability. As an alternative to sodium bicarbonate, it used in the treatment of metabolic acidosis. It acts as a precursor for the preparation of polymers, oxazolones and oxazolidines.
Preparation
Tris(hydroxymethyl)aminomethane is prepared industrially by the exhaustive condensation of nitromethane with formaldehyde under basic conditions (i.e. repeated Henry reactions) to produce the intermediate (HOCH2)3CNO2, which is subsequently hydrogenated to give the final product.
Definition
ChEBI: Tris is a primary amino compound that is tert-butylamine in which one hydrogen attached to each methyl group is replaced by a hydroxy group. A compound widely used as a biological buffer substance in the pH range 7--9; pKa = 8.3 at 20 ℃; pKa = 7.82 at 37 ℃. It has a role as a buffer. It is a triol and a primary amino compound. It is a conjugate base of a member of Htris.
Manufacturing Process
Nitromethane is reacted with formaldehyde to give tris(hydroxymethyl)nitromethane in an initial step. This intermediate may be reduced by catalytic hydrogenation (US Patent 2,174,242) or by electrolytic reduction (US Patent 2,485,982).
brand name
Tham (Hospira).
Therapeutic Function
Antacid
General Description
Tris(hydroxymethyl)aminomethane is an established basimetric standard and buffer used in biochemistry and molecular biology. It may be used by itself as a buffer or as a component of mixed buffer formulations, such as Tris-EDTA (TE) buffer, Tris-acetate-EDTA (TAE) buffer, Tris-borate-EDTA (TBE) buffer, etc. It is pure, essentially stable, relatively non-hygroscopic and has a high equivalent weight.
Biochem/physiol Actions
Trizma base, commonly known as tris(hydroxymethyl)aminomethane is widely used as a biological buffer or as a component of buffer formulations like- Tris-EDTA (TE) buffer, Tris magnesium buffer or Tris-acetate-EDTA (TAE) buffer. It enhances the membrane permeability of cell membranes.
Metabolism
Not Available
Storage
Store at RT
Purification Methods
Tris(hydroxymethyl)aminomethane can ordinarily be obtained in highly pure form suitable for use as an acidimetric standard. If only impure material is available, it should be crystallised from 20% EtOH, aqueous MeOH (m 171.1o) or isopropanol (m 172-173o). Dry it in a vacuum desiccator over P2O5 or CaCl2. Alternatively, it is dissolved in twice its weight of water at 55-60o, filtered, concentrated to half its volume and poured slowly, with stirring, into about twice its volume of EtOH. The crystals which separate on cooling to 3-4o are filtered off, washed with a little MeOH, air dried by suction, then finally ground and dried in a vacuum desiccator over P2O5. It has also been recrystallised from water, MeOH or aqueous MeOH, and vacuum dried at 80o for 2 days. [Beilstein 4 H 303, 4 III 857, 4 IV 1903.]
Properties of Tris(hydroxymethyl)aminomethane
| Melting point: | 167-172 °C(lit.) |
| Boiling point: | 219-220 °C10 mm Hg(lit.) |
| Density | 1,353 g/cm3 |
| vapor pressure | 0.0267 hPa (20 °C) |
| refractive index | 1.4170 (estimate) |
| Flash point: | 219-220°C/10mm |
| storage temp. | 20-25°C |
| solubility | H2O: 4 M at 20 °C, clear, colorless |
| form | crystalline |
| color | white |
| PH | 10.5-12.0(4 m in water, 25 °C) |
| pka | 8.1(at 25℃) |
| PH Range | 7 - 9 |
| Water Solubility | 550 g/L (25 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.10 λ: 280 nm Amax: 0.08 |
| Merck | 14,9772 |
| BRN | 741883 |
| Stability: | Stable. Incompatible with bases, strong oxidizing agents. Protect from moisture. |
| CAS DataBase Reference | 77-86-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,3-Propanediol, 2-amino-2-(hydroxymethyl)-(77-86-1) |
| EPA Substance Registry System | Tris(hydroxymethyl)aminomethane (77-86-1) |
Safety information for Tris(hydroxymethyl)aminomethane
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tris(hydroxymethyl)aminomethane
| InChIKey | LENZDBCJOHFCAS-UHFFFAOYSA-N |
Tris(hydroxymethyl)aminomethane manufacturer
JSK Chemicals
Techno Pharmchem
PNR Impex
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Tris-(hydroxymethyl)-aminomethane 98%View Details
Tris-(hydroxymethyl)-aminomethane 98%View Details -
 Tris(hydroxymethyl)aminomethane CAS 77-86-1View Details
Tris(hydroxymethyl)aminomethane CAS 77-86-1View Details
77-86-1 -
 Tris(hydroxymethyl)aminomethane CAS 77-86-1View Details
Tris(hydroxymethyl)aminomethane CAS 77-86-1View Details
77-86-1 -
 Tris Buffer Superior for molecular biology CAS 77-86-1View Details
Tris Buffer Superior for molecular biology CAS 77-86-1View Details
77-86-1 -
 Tris Buffer for HPLC CAS 77-86-1View Details
Tris Buffer for HPLC CAS 77-86-1View Details
77-86-1 -
 Tris Buffer AR CAS 77-86-1View Details
Tris Buffer AR CAS 77-86-1View Details
77-86-1 -
 Tris(hydroxymethyl)aminomethane AR ACS molecular biology 99.9% CAS 77-86-1View Details
Tris(hydroxymethyl)aminomethane AR ACS molecular biology 99.9% CAS 77-86-1View Details
77-86-1 -
 2-Amino-2-Hydroxymethyl-1,3-Propanediol, C4H11NO3, Purity: 99View Details
2-Amino-2-Hydroxymethyl-1,3-Propanediol, C4H11NO3, Purity: 99View Details
77-86-1
