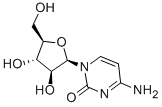
Cytidine
Synonym(s):Cytosine β-D -riboside;Cytosine-1-β-D -ribofuranoside
- CAS NO.:65-46-3
- Empirical Formula: C9H13N3O5
- Molecular Weight: 243.22
- MDL number: MFCD00006545
- EINECS: 200-610-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 18:23:36
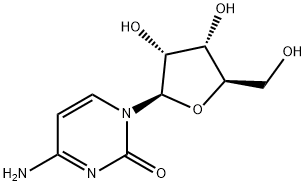
What is Cytidine?
Description
Cytidine is a pyrimidine nucleoside that is composed of the pyrimidine base cytosine attached to the sugar ribose. As a constituent of RNA, cytidine pairs with guanine, and it is a precursor of uridine. Cytidine can incur several modifications in mRNA, including methylation and acetylation, that function to regulate translation. Cytidine can also be formylated to 5-formylcytidine in mitochondrial methionine transfer RNA (tRNAMet).
Chemical properties
Cytidine is a component of RNA. It is a white water-soluble solid. which is only slightly soluble in ethanol.There are a variety of cytidine analogs with potentially useful pharmacology.
The Uses of Cytidine
Cytidine is a nucleoside molecule that is formed when it is attached to a ribose ring, cytidine is a component of RNA. It was isolated from yeast nucleic acid. It can increases cell membrane phospholipids. Cytidine is used to synthesize enzyme inhibitors, antiviral agents, and anticancer agents. And also used as constituent of nucleic acids.
Definition
ChEBI: cytidine is a pyrimidine nucleoside in which cytosine is attached to ribofuranose via a beta-N(1)-glycosidic bond. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It derives from a cytosine.The derivednucleotides, cytidine mono-, di-, andtriphosphate (CMP, CDP, and CTP respectively)participate in various biochemicalreactions, notably inphospholipid synthesis.
What are the applications of Application
Cytidine has been used as a non-essential aminoacid component of minimal essential medium (MEM) to analyse interspecies somatic cell nucleus transfer (iSCNT)-derived blastocysts. It has also been used as a supplement in the culture medium of HeLa cells, to study the effects of cytidine addition on rods and rings (RR) induced by glutamine deprivation.
Cytidine has been used as a component to prepare ribonucleoside stock solution to assess its effects on the anaerobic growth of several Bacillus mojavensis strains.
General Description
White crystalline powder.
Air & Water Reactions
Cytidine is hygroscopic. Water soluble.
Reactivity Profile
Cytidine is incompatible with strong oxidizing agents. . Will react as a weak base.
Fire Hazard
Flash point data for Cytidine are not available; however, Cytidine is probably combustible.
Mechanism
While the complete mechanism of cytidine′s action remains somewhat elusive, it is believed to have a significant influence on protein synthesis and DNA replication regulation. This is thought to occur through interactions with specific proteins, including transcription factors and DNA polymerases, which in turn modulate gene expression and DNA replication. Furthermore, cytidine is believed to play a part in the generation of new RNA molecules and the repair of damaged DNA. As a precursor for the synthesis of crucial molecules like uridine and cytidine triphosphate, cytidine′s involvement extends to the production of energy, adding yet another layer to its importance in the biological realm.
Purification Methods
Cytidine crystallises from 90% aqueous EtOH. It has also been converted to sulfate by dissolving (~200mg) in a solution of EtOH (10mL) containing H2SO4 (50mg), whereby the salt crystallises out. It is collected, washed with EtOH and dried for 5hours at 120o/0.1mm. The sulfate has m 225o. The free base is obtained by shaking the salt solution with a weak ion-exchange resin, filtering, evaporating and recrystallising the residue from EtOH as before. [Fox & Goodman J Am Chem Soc 73 3256 1956, Fox & Shugar Biochim Biophys Acta 9 369 1952; see Prytsas & Sorm in Synthetic Procedures in Nucleic Acid Chemistry (Zorbach & Tipson Eds) Vol 1 404 1973.] [Beilstein 25 III/IV 3667.]
Properties of Cytidine
| Melting point: | 210-220 °C (dec.) (lit.) |
| Boiling point: | 386.09°C (rough estimate) |
| alpha | 31.5 º (c=0.6, H2O 25 ºC) |
| Density | 1.3686 (rough estimate) |
| refractive index | 34 ° (C=0.7, H2O) |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL |
| form | powder |
| pka | 4.22, 12.5(at 25℃) |
| color | White to almost white |
| optical activity | [α]20/D +33°, c = 2 in H2O |
| Water Solubility | SOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,2786 |
| BRN | 89173 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Protect from moisture. |
| CAS DataBase Reference | 65-46-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Cytidine(65-46-3) |
| EPA Substance Registry System | Cytidine (65-46-3) |
Safety information for Cytidine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cytidine
Cytidine manufacturer
Jaiswal S Cyber Shop
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Cytidine 98%View Details
Cytidine 98%View Details -
 Cytidine extrapure CAS 65-46-3View Details
Cytidine extrapure CAS 65-46-3View Details
65-46-3 -
 Cytidine, 99% CAS 65-46-3View Details
Cytidine, 99% CAS 65-46-3View Details
65-46-3 -
 Cytidine 98% CAS 65-46-3View Details
Cytidine 98% CAS 65-46-3View Details
65-46-3 -
 Cytidine, 1kg, ChinaView Details
Cytidine, 1kg, ChinaView Details
65-46-3 -
 White Jcssuper 65-46-3 Cytidine For Biochemistry 1 Gm., Packaging Type: BottleView Details
White Jcssuper 65-46-3 Cytidine For Biochemistry 1 Gm., Packaging Type: BottleView Details
65-46-3 -
 White Cytidine (CAS Number: 65-46-3)View Details
White Cytidine (CAS Number: 65-46-3)View Details
65-46-3 -
 Cytidine . ., Bio-Tech Grade, 1 KgView Details
Cytidine . ., Bio-Tech Grade, 1 KgView Details
65-46-3
