2'-Deoxycytidine monohydrate
- CAS NO.:951-77-9
- Empirical Formula: C9H13N3O4
- Molecular Weight: 227.22
- MDL number: MFCD00006547
- EINECS: 213-454-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:04
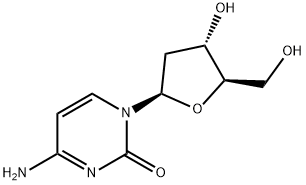
What is 2'-Deoxycytidine monohydrate?
Description
2'-Deoxycytidine is a deoxyribonucleotide that is used in the synthesis of DNA. 2'-Deoxycytidine has been shown to inhibit the kinase activity of IL-2 receptor and Toll-like receptor, which are proteins that regulate the immune response. 2'-Deoxycytidine also inhibits DNA polymerase activity and thermal expansion, which may make it a good candidate as an anticancer drug.
Chemical properties
White crystalline powder
The Uses of 2'-Deoxycytidine monohydrate
A deoxyribonucleoside
The Uses of 2'-Deoxycytidine monohydrate
2'-Deoxycytidine (deoxyC) is one of the deoxynucleosides which after phosphorylation to dCTP is used to synthesis DNA via various DNA polymerases or reverse transcriptases. 2'-Deoxycytidine (deoxyC) is the substrate for deoxycytidine deaminase (EC 3.5.4.14) which converts it into 2?-deoxyuridine. 2?-Deoxycytidine is phosphorylated to the nucleotide dCMP by the enzyme deoxycytidine kinase (DCK).
What are the applications of Application
2′-Deoxy Cytidine is A deoxyribonucleoside as Eg5 kinesin modulator
Definition
ChEBI: A pyrimidine 2'-deoxyribonucleoside having cytosine as the nucleobase.
General Description
2′-Deoxycytidine (deoxyC) is one of the deoxynucleosides containing cytosine as the nucleobase. It is found in the blood, feces, and urine.
Biological Activity
2-deoxycytidine is a cytidine analog [1].2-deoxycytidine prevents dna methylation by incorporating itself into newly synthesizing dna strand. 2-deoxycytidine also binds to dna methyltransferase irreversibly and hinders its activity. thus, 2-deoxycytidine was approved as the most efective demethylating agent for the treatment of cancer [1].2-deoxycytidine at clinically achievable and nontoxic concentrations (≥ 100 μmol/l) protected normal bone marrow progenitor cells against the inhibitory effects of co-administered, high concentrations of 3’-azido-3’-deoxythymidine (azt) (≥ 10 μmol/l). in normal bone marrow mononuclear cells (bmmc), 2-deoxycytidine also significantly corrected azt-mediated depletion of intracellular thymidine triphosphate and 2-deoxycytidine triphosphate levels. furthermore, 2-deoxycytidine reduced the intracellular accumulation of azt triphosphate and its dna incorporation in bmmc [2].in a rat model of myocardial infarction induced by ligating left anterior descending coronary artery, human umbilical cord mesenchymal stem cells treated with 2-deoxycytidine (5, 10, 20 and 40 μm) before transplantation to the left ventricular wall immediately after ligation significantly improved the cardiac systolic and diastolic functions, and pumping ability. fibrotic area and left ventricular wall thickness were also significantly improved [1].[1]. ali s r, ahmad w, naeem n, et al. small molecule 2'-deoxycytidine differentiates human umbilical cord-derived mscs into cardiac progenitors in vitro and their in vivo xeno-transplantation improves cardiac function. molecular and cellular biochemistry, 2020, 470(1-2): 99-113.[2]. bhalla k, birkhofer m, li g r, et al. 2'-deoxycytidine protects normal human bone marrow progenitor cells in vitro against the cytotoxicity of 3'-azido-3'-deoxythymidine with preservation of antiretroviral activity. blood, 1989, 74(6): 1923-1928.
Biochem/physiol Actions
2′-Deoxycytidine (deoxyC) forms dCTP upon phosphorylation which is used to synthesis DNA via various DNA polymerases or reverse transcriptases. DeoxyC is the substrate for deoxycytidine deaminase (EC 3.5.4.14) which converts it into 2′-deoxyuridine. DeoxyC is phosphorylated to the nucleotide dCMP by the enzyme deoxycytidine kinase (DCK). DeoxyC serves as a potential head and neck cancer marker.
Safety Profile
Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Purification Methods
Purify 2'-deoxycytidine by recrystallisation from MeOH/Et2O or EtOH and dry it in air. [NMR: Miles J Am Chem Soc 85 1007 1963, UV: Fox & Shugar Biochim Biophys Acta 9 369 1952.] The hydrochloride crystallises from H2O/EtOH and has m 174o(dec, 169-173o). [Walker & Butler Can J Chem 34 1168 1956.] The picrate has m 208o(dec). [Fox et al. J Am Chem Soc 83 4066 1961, Beilstein 25 III/IV 3662.]
Structure and conformation
2'-Deoxycytidine monohydrate (dCMP) is a nucleoside phosphate in being comprised of a deoxyribonucleoside and one phosphate group. It has a deoxyribose as its sugar constituent with one phosphate group attached. Its nucleoside contains a pyrimidine base, i.e., a cytosine attached to the deoxyribose sugar. It has only one phosphate group attached to the nucleoside. Its conjugate acid form is deoxycytidylic acid, whereas its conjugate base form is deoxycytidylate. dCMP, instead of having a hydroxyl group on the 2′ carbon of the sugar component as it is in Cytidine monophosphate (CMP), has it reduced to a hydrogen atom (thus, deoxy- in its name). dCMP is one of the monomeric units that constitute DNA, whereas CMP is one of the monomeric units that make up RNA.
Properties of 2'-Deoxycytidine monohydrate
| Melting point: | 209-211 °C(lit.) |
| Boiling point: | 368.93°C (rough estimate) |
| Density | 1.3171 (rough estimate) |
| refractive index | 59 ° (C=1, H2O) |
| storage temp. | -20°C |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | Crystalline Powder |
| pka | 14.03±0.60(Predicted) |
| color | White |
| Water Solubility | Soluble in water, DMSO. |
| BRN | 87567 |
| InChI | InChI=1S/C9H13N3O4/c10-7-1-2-12(9(15)11-7)8-3-5(14)6(4-13)16-8/h1-2,5-6,8,13-14H,3-4H2,(H2,10,11,15)/t5-,6+,8+/m0/s1 |
| CAS DataBase Reference | 951-77-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Deoxycytidine(951-77-9) |
| EPA Substance Registry System | Cytidine, 2'-deoxy- (951-77-9) |
Safety information for 2'-Deoxycytidine monohydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for 2'-Deoxycytidine monohydrate
| InChIKey | CKTSBUTUHBMZGZ-SHYZEUOFSA-N |
| SMILES | OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 2-Deoxycytidine CAS 951-77-9View Details
2-Deoxycytidine CAS 951-77-9View Details
951-77-9 -
 2-Deoxycytidine extrapure CAS 951-77-9View Details
2-Deoxycytidine extrapure CAS 951-77-9View Details
951-77-9 -
 2'-Deoxycytidine CAS 951-77-9View Details
2'-Deoxycytidine CAS 951-77-9View Details
951-77-9 -
 2′-Deoxycytidine CAS 951-77-9View Details
2′-Deoxycytidine CAS 951-77-9View Details
951-77-9 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
