11-KETO-BETA-BOSWELLIC ACID
Synonym(s):11-Oxo-β-boswellic acid;3α-Hydroxy-11-oxours-12-en-24-oic acid;KBA
- CAS NO.:17019-92-0
- Empirical Formula: C30H46O4
- Molecular Weight: 470.68
- MDL number: MFCD06656312
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17
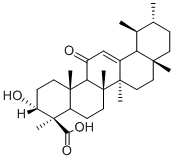
What is 11-KETO-BETA-BOSWELLIC ACID?
The Uses of 11-KETO-BETA-BOSWELLIC ACID
A constitutent of frankincense (olibanum) with anti-inflammatory properties. It has been shown to trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells.
Biological Activity
37.9 μm: blocks a2780 (cis-platin resistant ovarian cancer cells) [1].11-keto-β-boswellic acid, known as kba, is a naturally occurring pentacyclic triterpene isolated from the gum resin of the tree boswellia serrata. kba is non-redox, specific leukotriene synthesis inhibitors through the inhibition of 5-lipoxygenase (5-lox) which has anti-arthritic and anti-inflammatory activities. 5-lox catalyzes essential fatty acids substrates into leukotrienes and a variety of other biologically active products. kba is a novel activator of nuclear factor erythroid-2-related factor 2 (nrf2), which protects against cerebral ischemic injury.
in vitro
kba, concentration dependently, decreased the formation of leukotriene b4 and the synthesis of all 5-lox products from endogenous arachidonic acid in rat peritoneal neutrophils. in contrast, kba exerted no remarkable effects on the 12-lipoxygenase and cyclooxygenase activities. [2].
in vivo
adult male sprague–dawley rats were injected kab intraperitoneally at a dose of 25 mg/kg for 48 hours. kab remarkably decreased infarct volumes as well as apoptotic cells at 1 h, and then increased neurologic scores when applied 48 h. moreover, posttreatment with kba induced the decrease of malondialdehyde levels and the increase of protein nrf2 and heme oxygenase-1 expression in brain tissues, indicating that the nrf2/ho-1 pathway was involved in the neuroprotection of kba against oxidative stress-induced ischemic injury [3].
References
[1]. csuk, r., barthel-niesen, a., barthel, a., schffer, r., & al-harrasi, a. 11-keto-boswellic acid derived amides and monodesmosidic saponins induce apoptosis in breast and cervical cancers cells. european journal of medicinal chemistry. 2015; 100: 98-105.
[2]. safayhi, h., mack, t. h. o. m. a. s., sabieraj, j. o. a. c. h. i. m., anazodo, m. i., subramanian, l. r., & ammon, h. p. boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. journal of pharmacology and experimental therapeutics. 1992; 261(3):1143-1146.
[3]. ding, y., chen, m., wang, m., li, y., & wen, a. posttreatment with 11-keto-β-boswellic acid ameliorates cerebral ischemia–reperfusion injury: nrf2/ho-1 pathway as a potential mechanism. molecular neurobiology. 2014; 52(3): 1430-1439.
Properties of 11-KETO-BETA-BOSWELLIC ACID
| Melting point: | 190~192℃ |
| Boiling point: | 591.8±50.0 °C(Predicted) |
| Density | 1.14±0.1 g/cm3(Predicted) |
| storage temp. | 4°C, protect from light |
| solubility | ≤5mg/ml in ethanol;25mg/ml in DMSO;25mg/ml in dimethyl formamide |
| form | neat |
| pka | 4.46±0.70(Predicted) |
| form | Solid |
| color | White |
| Water Solubility | insoluble in water |
Safety information for 11-KETO-BETA-BOSWELLIC ACID
Computed Descriptors for 11-KETO-BETA-BOSWELLIC ACID
| InChIKey | YIMHGPSYDOGBPI-IQQSWPBXSA-N |
11-KETO-BETA-BOSWELLIC ACID manufacturer
GK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 11-Keto β boswellic acid 17019-92-0 >98%View Details
11-Keto β boswellic acid 17019-92-0 >98%View Details
17019-92-0 -
 11-Keto-β-boswellic acid CAS 17019-92-0View Details
11-Keto-β-boswellic acid CAS 17019-92-0View Details
17019-92-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
