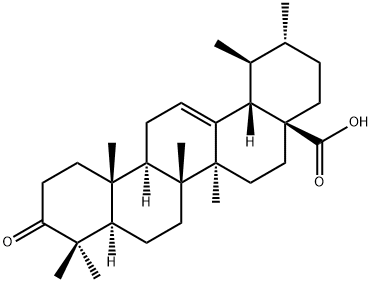
Ursolic acid
Synonym(s):3β-Hydroxy-12-ursen-28-ic acid;3β-Hydroxy-urs-12-en-28-oic acid, CG7, PTP1B Inhibitor VII, PTP Inhibitor XXVII, PTPN2/TCPTP Inhibitor II, SHP2 Inhibitor IV;3β-Hydroxy-urs-12-en-28-oic acid, CG7, PTPN2/TCPTP Inhibitor II, SHP2 Inhibitor IV, PTP1B Inhibitor VII, PTP Inhibitor XXVII;Ursolic Acid - CAS 77-52-1 - Calbiochem
- CAS NO.:77-52-1
- Empirical Formula: C30H48O3
- Molecular Weight: 456.7
- MDL number: MFCD00009621
- EINECS: 201-034-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
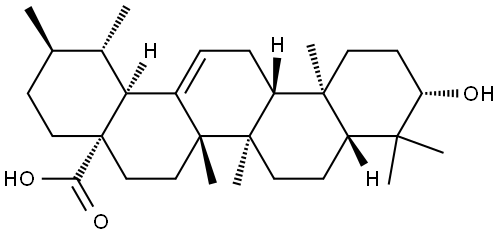
What is Ursolic acid?
Chemical properties
white to light yellow crystal powde
The Uses of Ursolic acid
Ursolic Acid is a Triterpene acid used in cosmetics, that also has STAT3 pathway inhibiting properties. It inhibits endothelial cell proliferation and migration (IC50=5 μM) and angiogenesis. Promotes skeletal muscle rejuvenation via enhanced SIRT1 expression. Induces apoptosis in malignant mesothelioma cells. Ursolic acid protects against muscle atrophy and boosts muscle growth (see also tomatidine T538500).
What are the applications of Application
Ursolic Acid is an antiangiogenic and antiproliferative agent
Definition
ChEBI: Ursolic acid is a pentacyclic triterpenoid that is urs-12-en-28-oic acid substituted by a beta-hydroxy group at position 3. It has a role as a plant metabolite and a geroprotector. It is a pentacyclic triterpenoid and a hydroxy monocarboxylic acid. It derives from a hydride of an ursane.
What are the applications of Application
ursolic acid helps maintain the look and feel of the skin, acts as a perfume and helps mask odor. Therapeutic benefits in skin care include anti-inflammatory activity. ursolic acid has demonstrated anti-microbial, anti-bacterial, and anti-fungal action as well.
General Description
Ursolic acid (UA) is a hydroxyl pentacyclic triterpenoic acid (HPTA), which exhibits anti-bacterial, anti-cancer, anti-oxidative and anti-inflammatory effects. It can also promote neuroregeneration after peripheral nerve injury. UA can enhance sleep duration by activating the GABAergic neurotransmitter system. [GABA= γ-aminobutyric acid]
Biochem/physiol Actions
Triterpenoid found in a variety of fruits, including apples. Cardioprotective and anti-tumor agent. Under study as a potential Alzheimer′s disease therapeutic due to its inhibitory effect on the interactions between amyloid-β and the CD36 receptor.
Anticancer Research
It is a triterpenoid, derived from basil, and suppresses the activation of NF-κB byinhibiting a kinase that activates NF-κB(IKK) (Aggarwal and Shishodia 2004). It suppresses tumorigenesis, tumor promotion, and angiogenesis by downregulationof the expression of lipoxygenases (LOX), MMP-9, and COX-2. It is reported toinduce apoptosis in breast cancer, melanoma, hepatoma, prostate cancer, andacute myelogenous leukemia by preventing replication of DNA, inhibition of proteintyrosine kinases, activation of caspases, induction of calcium release, anddownregulating apoptosis gene cellular inhibitor. Ursolic acid inhibits IκBαkinase activity, degradation of IκBα, phosphorylation, NF-κB-dependent reportergene expression, p65 nuclear translocation, and p65 phosphorylation. Inhibitionof NF-κB leads to the reduction of cyclin D1, COX-2, and MMP9 expression.This downregulates STAT-3 activation and its regulated gene products such ascyclin D1, Mcl-1, Bcl-xL, survivin, Bcl-2, and VEGF. It also induces the expressionof tyrosine phosphatase SHP-1 protein and of mRNA (Aggarwal et al. 2008;Hsu et al. 2004).
Side Effects
Adverse effects of Ursolic acid include reduction of sperm viability and inhibition of spermatogenesis, leading to infertility. Ursolic acid is capable of inducing endothelial cell death at concentrations above 12.5uM, and the mechanism of death is related to apoptosis. In addition, it also appears to cause DNA strand breaks.
Cytotoxicity
IC50 (μg/mL): 6.7 (518A2), 5.3 (A2780), 7(A549), 6.4 (FaDu), 4.8 (HT29), 5.8(MCF-7), 8.5 (NIH3T3) (Wiemann et al.2016)
IC50 (μg/mL): 12.38 (MGC-803),17.12(HCT-116), 13.39 (T24), 13.81(HepG2), 16.36 (A549), >45.7 (HL-7702)(Hua et al. 2015).
Properties of Ursolic acid
| Melting point: | 292 °C (dec.) (lit.) |
| Boiling point: | 502.79°C (rough estimate) |
| alpha | 59 º (c=0.3, pyridine) |
| Density | 1.0261 (rough estimate) |
| refractive index | 1.4940 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (up to 25 mg/ml with warming) |
| form | Crystalline Powder or Needles |
| pka | 4.68±0.70(Predicted) |
| color | White to off-white |
| Water Solubility | insoluble |
| Merck | 14,9890 |
| BRN | 2228563 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months. |
| CAS DataBase Reference | 77-52-1(CAS DataBase Reference) |
| EPA Substance Registry System | Urs-12-en-28-oic acid, 3-hydroxy-, (3.beta.)- (77-52-1) |
Safety information for Ursolic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ursolic acid
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran

You may like
-
 77-52-1 Ursolic acid 98%View Details
77-52-1 Ursolic acid 98%View Details
77-52-1 -
 77-52-1 98%View Details
77-52-1 98%View Details
77-52-1 -
 Ursolic acid 95.00% CAS 77-52-1View Details
Ursolic acid 95.00% CAS 77-52-1View Details
77-52-1 -
 Ursolic acid, 90% CAS 77-52-1View Details
Ursolic acid, 90% CAS 77-52-1View Details
77-52-1 -
 Ursolic Acid CAS 77-52-1View Details
Ursolic Acid CAS 77-52-1View Details
77-52-1 -
 Ursolic acid 98%View Details
Ursolic acid 98%View Details
77-52-1 -
 Ursolic acid >98% (HPLC) CAS 77-52-1View Details
Ursolic acid >98% (HPLC) CAS 77-52-1View Details
77-52-1 -
 Ursolic acid CAS 77-52-1View Details
Ursolic acid CAS 77-52-1View Details
77-52-1
