Linagliptin
- CAS NO.:668270-12-0
- Empirical Formula: C25H28N8O2
- Molecular Weight: 472.54
- MDL number: MFCD14635356
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
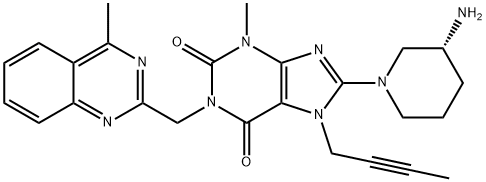
What is Linagliptin?
Description
Linagliptin (trade names Tradjenta and Trajetna) is an inhibitor of dipeptidyl peptidase-4 (DPP-4) that was approved by the U.S. FDA in May 2011 for the treatment of Type 2 diabetes along with diet and exercise. Linagliptin (BI-1356) has been described as a potent highly selective, slow-off rate and long acting inhibitor of DPP-4. Linagliptin arose from optimization efforts of xanthine-based DPP-4 inhibitors with the initial lead identified from an HTS campaign. After optimizing the activity of the initial micromolar lead, two issues that needed to be addressed were activity for hERG and muscarinic receptor M1. Introduction of a butynyl group at the N7 position of the xanthine ring gave much reduced M1 affinity with no measureable hERG activity. Linagliptin inhibits DPP-4 with an IC50=1 nM and is highly selective (>10,000-fold) against DPP-8 and DPP-9. Linagliptin shows no interactions with CYPs up to 50 mM. The described synthesis of linagliptin starts with 8-bromoxanthine, which is alkylated at the N-7 position to introduce the butyne group, followed by alkylation of the N-1 group to introduce the methyl-quinazoline group. Displacement of the bromide with (R)-Boc-3-amino-piperidine followed by deprotection gives linagliptin. When administered to db/db mice orally, linagliptin dose dependently reduced glucose excursion from 0.1 mg/kg (15% inhibition) to 1 mg/kg (66% inhibition).
Originator
Boehringer Ingelheim (United States)
The Uses of Linagliptin
Labeled Linagliptin, intended for use as an internal standard for the quantification of Linagliptin by GC- or LC-mass spectrometry.
The Uses of Linagliptin
A novel potent and selective dipeptidyl peptidase-4 (DPP-4) inhibitor with potential use in the treatment of type 2 diabetes.
The Uses of Linagliptin
dipeptidypeptidase inhibitor, antidiabetic
The Uses of Linagliptin
highly potent CD26 inhibitor
Definition
ChEBI: A xanthine that is 7H-xanthine bearing (4-methylquinazolin-2-yl)methyl, methyl, but-2-yn-1-yl and 3-aminopiperidin-1-yl substituents at positions 1, 3, 7 and 8 respectively (the R-enantiomer). Used for treatment of type I diabetes.
Indications
Linagliptin is indicated for the treatment of type II diabetes in addition to diet and exercise. It should not be used to treat type I diabetes or in diabetic ketoacidosis. An extended-release combination product containing empagliflozin, linagliptin, and metformin was approved by the FDA in January 2020 for the improvement of glycemic control in adults with type 2 diabetes mellitus when used adjunctively with diet and exercise.
Background
Linagliptin is a DPP-4 inhibitor developed by Boehringer Ingelheim for the treatment of type II diabetes . Linagliptin differs from other DPP-4 inhibitors in that it has a non-linear pharmacokinetic profile, is not primarily eliminated by the renal system, and obeys concentration dependant protein binding. Linagliptin was approved by the FDA on May 2, 2011.
brand name
Tradjenta
Pharmacokinetics
A 5mg oral dose of linagliptin results in >80% inhibition of dipeptidyl peptidase 4 (DPP-4) for ≥24 hours. Inhibition of DPP-4 increases the concentration of glucagon-like peptide 1 (GLP-1), leading to decreased glycosylated hemoglobin and fasting plasma glucose.
Clinical Use
Type 2 diabetes mellitus
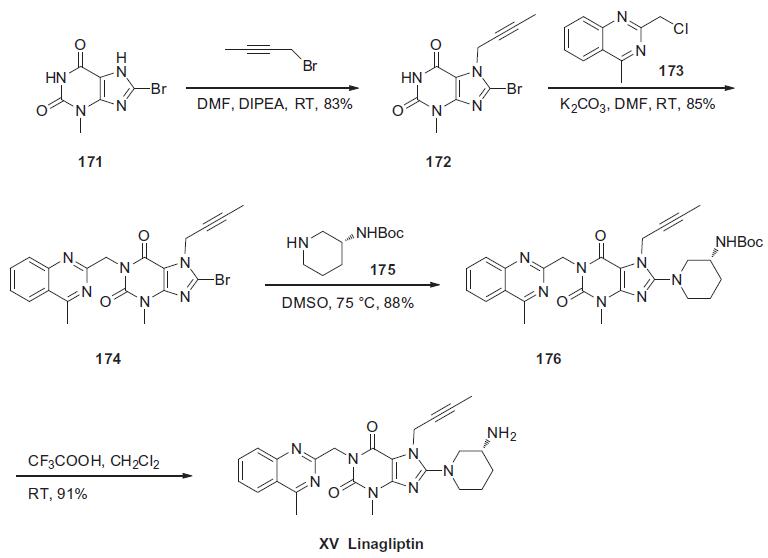
Synthesis
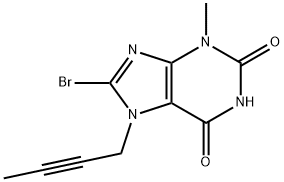
The synthesis of linagliptin began from commercially available 8-bromo-3-methylxanthine (171). Sequential alkylations of guanine derivative 171 at N-7 with butyn-2-yl bromide in the presence of N,N-diisopropylethylamine and N-1 with 2- (chloromethyl)-4-methylquinazoline (173) in the presence of potassium carbonate, yielded N1,N7-dialkylated xanthine 174 in 85% yield. This material was further condensed with (R)-3-Bocaminopiperidine (175) in the presence of potassium carbonate to give aminopurine dione 176 in 88% yield. Finally, the primary amine of 176 was liberated with trifluoroacetic acid in methylene chloride to produce linagliptin (XV) in 91% yield.
Metabolism
An oral dose of linagliptin is excreted primarily in the feces. 90% of an oral dose is excreted unchanged in the urine and feces. The predominant metabolite in the plasma is CD1790 and the predominant metabolite recovered after excretion was M489(1). Other metabolites are produced through oxidation, oxidative degradation, N-acetylation, glucuronidation, and cysteine adduct formation. Other metabolites have been identified through mass spectrometry though no structures were determined. Metabolism of linagliptin is mediated by cytochrome P450 3A4, aldo-keto reductases, and carbonyl reductases.
Absorption
Oral bioavailability of linagliptin is 30%.
Storage
+4°C
Toxicity
No dosage adjustment is necessary based on race, age, weight, sex, renal impairment, or hepatic impairment.
Studies of efficacy and safety in pediatric populations were not included in the original drug approval but recent clinical trials show linagliptin to be well tolerated in patients 10 to 18 years old.
Animal studies showed an increased risk of lymphoma in female rats at over 200 times the clinical dose. Aside from this effect, linagliptin was not shown to be mutagenic, clastogenic, or have an effect on fertility.
Properties of Linagliptin
| Melting point: | 202 ºC |
| Boiling point: | 661.2±65.0 °C(Predicted) |
| Density | 1.39 |
| storage temp. | Refrigerator |
| solubility | Chloroform (Sparingly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 10.01±0.20(Predicted) |
| color | White to Orange |
Safety information for Linagliptin
Computed Descriptors for Linagliptin
| InChIKey | LTXREWYXXSTFRX-QGZVFWFLSA-N |
| SMILES | N1(CC#CC)C2=C(N(C)C(=O)N(CC3=NC(C)=C4C(=N3)C=CC=C4)C2=O)N=C1N1CCC[C@@H](N)C1 |
Linagliptin manufacturer
Mekanar Pharma Private Limited
DR ACHARYA LABORATORIES PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran







You may like
-
 668270-12-0 98%View Details
668270-12-0 98%View Details
668270-12-0 -
 Linagliptin 97%View Details
Linagliptin 97%View Details -
 Linagliptin 99%View Details
Linagliptin 99%View Details -
 LINAGLIPTIN 98%View Details
LINAGLIPTIN 98%View Details -
 Linagliptin 99%View Details
Linagliptin 99%View Details -
 Linagliptin 98%View Details
Linagliptin 98%View Details -
 Linagliptin 98% (HPLC) CAS 668270-12-0View Details
Linagliptin 98% (HPLC) CAS 668270-12-0View Details
668270-12-0 -
 CAS 668270 12 0 LINAGLIPTIN, Packaging Size: 1KG, 2.5 mgView Details
CAS 668270 12 0 LINAGLIPTIN, Packaging Size: 1KG, 2.5 mgView Details
668270-12-0
