Bexarotene
Synonym(s):4-(1-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydronaphthalen-2-yl)vinyl)benzoic acid;4-[1-(5,6,7,8-Tetrahydro-3,5,5,8,8-pentamethyl-2-naphthalenyl)ethenyl]benzoic acid;Bexarotene - CAS 153559-49-0 - Calbiochem;LGD-1069;SR-11247
- CAS NO.:153559-49-0
- Empirical Formula: C24H28O2
- Molecular Weight: 348.48
- MDL number: MFCD00932428
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-06 15:14:14

What is Bexarotene?
Description
Bexarotene was launched in the US for the treatment of manifestations of cutaneous T-cell lymphoma in patients who are refractory to at least one prior systemic therapy. The four step synthesis of bexarotene involves a double Friedel-Craft alkylation of toluene with 2,5-dichloro-2,5-dimethylhexane followed by acylation with monomethylterephthalic acid chloride, then Wittig methylidenation. Bexarotene is the first retinoid X receptor (RXR) agonist to be selective versus retinoid A receptors (RAR). Its activation of the three RXRα, β, γ isoforms induces cell differentiation and apoptosis and inhibits cell proliferation in several models of cancer. In phase ll/lll clinical trials, 48% of patients with refractory or persistent early-stage cutaneous T-cell lymphoma achieved a complete or partial response when treated with 300 mg/m2/day of bexarotene. It was shown in phase I trials that this second-generation retinoid was substantially less toxic than the broad-spectrum or RARselective retinoids.
Description
Bexarotene is an anticancer agent developed by Ligand Pharmaceuticals and marketed under the trade name Targretin. It was approved by the FDA for treating cutaneous T-cell lymphoma in 1999. The rights to bexarotene were purchased by Eisai in 2006. Recently, G. Landreth and co-workers showed that?bexarotene removes amyloid-β protein from the brains of mice with Alzheimer’s disease?(AD) and thus restores their memory. Whether this finding can be applied to AD in humans remains to be seen.
Description
Bexarotene is an agonist of retinoid X receptors (RXRs; EC50s = 28, 25, and 20 nM for RXRα, RXRβ, and RXRγ, respectively, in reporter assays). It is selective for RXRs over retinoic acid receptors (RARs; EC50s = >10 μM for RARα, RARβ, and RARγ). Bexarotene (10 μM) induces apoptosis in MJ, HuT 78, and HH cutaneous T cell lymphoma (CTCL) cells, as well as inhibits lung metastasis and angiogenesis in A549 and MDA-MB-231 mouse xenograft models when administered at a dose of 100 mg/kg per day. It reduces increased brain interstitial fluid levels of amyloid-β (1-40) (Aβ40) and Aβ42 in the APP/PS1 transgenic mouse model of Alzheimer’s disease. It also reduces viral load in the culture supernatant of Vero E6 cells infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; EC90 = 9.4 μM) and inhibits SARS-CoV-2 replication in a plaque reduction assay (EC50 = 2.01 μM). Formulations containing bexarotene have been used in the treatment of CTCL.
Chemical properties
White Solid
Originator
Ligand (US)
The Uses of Bexarotene
Bexarotene is used as a Synthetic retinoid analog with specific affinity for the retinoid X receptor, an antineoplastic agent, already approved as an oral antineoplastic agent for cutaneous T cell lymphoma and being investigated against other cancers. A study has found that bexarotene in a mouse Alzheimer?s model lowered the most toxic form of β-amyloid peptide and increased cognitive ability.
The Uses of Bexarotene
Used as an antineoplastic. A selective retinoid X receptor (RXR) agonist
What are the applications of Application
Bexarotene is a selective agonist of retinoid X receptors
Indications
Used orally for the treatment of skin manifestations of cutaneous T-cell lymphoma (CTCL) in patients who are refractory to at least one prior systemic therapy. Also used topically for the treatment of skin lesions in early (stage IA and IB) CTCL in patients who experience refractory or persistent disease with the use of other therapies or are intolerant of other therapies.
Background
Bexarotene (Targretin) is an antineoplastic agent indicated by the FDA for Cutaneous T cell lymphoma. It has been used off-label for lung cancer, breast cancer, and Kaposi's sarcoma.
Indications
Bexarotene (Targretin) belongs to a subclass of retinoids that selectively bind to and activates retinoid X receptors (RXRs), which have biological properties distinct from those of RARs. In vitro, bexarotene exerts antiproliferative effects on some tumor lines of hematopoietic and squamous cell origin.
Definition
ChEBI: Bexarotene is a retinoid, a member of benzoic acids and a member of naphthalenes. It has a role as an antineoplastic agent.
Manufacturing Process
(a) Methyl [4-(5,6,7,8-tetrahydro-3,5,5,8,8-pentamethyl-2-naphthalenyl)
carbonyl]benzoate (1):
To a suspension of aluminum chloride (1.10 g, 8.25 mmol) in 30 mL of 1,2-
dichloroethane under argon at room temperature was added a solution of
1,2,3,4-tetrahydro-1,1,4,4,6-pentamethylnaphthalene (1.52 g, 7.5 mmol)
(Kagechika, H. et al., J. Med. Chem, 31:2182 (1988)) and 4-
carbomethoxybenzoyl chloride (1.57 g, 7.87 mmol) in 15 mL of 1,2-dichloroethane. The reaction mixture was stirred overnight and poured onto
ice water and extracted with 40% ethyl acetate/hexane. The combined
organic layers were washed with saturated aqueous NaHCO3and brine. The
solution was dried over anhydrous MgSO4, filtered and concentrated to afford
a brown solid (2.56 g). Flash chromatography (60% dichloromethane/hexane)
yielded the desired product (1) as a white, crystalline solid (1.733 g, 64 %):
m.p. 146°-149°C; Rf 0.11 (50% CH2Cl2/hexane). The structure of the product
was also confirmed using IR, 1H NMR and mass spectroscopy.
(b) [4-(5,6,7,8-Tetrahydro-3,5,5,8,8-pentamethyl-2-naphthalenyl)
carbonyl]benzoic acid (2):
To a suspension of the ester (1) (0.120 g, 0.329 mmol) in 75% aqueous
methanol (2 mL) was added potassium hydroxide (0.055 g). The reaction
mixture was stirred at 60°C for 1 h during which time the material dissolved.
The solution was cooled to room temperature, acidified with 1 N aqueous
hydrochloric acid, and then extracted with 80% ethyl acetate/hexane. The
combined organic layers were dried over anhydrous MgSO4, filtered and
concentrated to afford a white solid (0.109 g). Recrystallization from
benzene/hexane afforded (2) as a white, crystalline solid (0.102 g, 89%):
m.p. 209°-212°C. The structure of the product was also confirmed using IR,
1H NMR and mass spectroscopy.
(c) Methyl 4-[1-(5,6,7,8-tetrahydro-3,5,5,8,8-pentamethyl-2-naphthalenyl)-1-
ethenyl]benzoate (3):
To a suspension of methyltriphenylphosphonium bromide (0.196 g, 0.55
mmol) in 1 mL of benzene under argon at room temperature was added a 0.5
M solution of potassium hexamethyldisilazide in toluene (1.2 mL, 0.6 mmol),
and the yellow solution was stirred for 5 min. A solution of keto-ester (1) (0.1
g, 0.274 mmol) in 1.5 mL of benzene was added and the orange solution was
stirred for 3 h at room temperature. The reaction mixture was filtered through
a plug of silica gel with 40% ethyl acetate/hexane. The filtrate was
concentrated to afford a solid. Flash chromatography (30%; 40%
dichloromethane/hexane) yielded the desired product (3) as a white solid
(0.077 g, 78%): m.p. 167°-168°C; Rf 0.4 (50% dichloromethane/hexane).
The structure of the product was also confirmed using IR, 1H NMR and mass
spectroscopy.
(d) [4-[1-(5,6,7,8-Tetrahydro-3,5,5,8,8-pentamethyl-2-naphthalenyl)-1-
ethenyl]benzoic acid (4):
To a suspension of the ester (3) (0.058 g, 0.16 mmol) in 75% aqueous
methanol (2 mL) was added one pellet of potassium hydroxide (0.1 g). The
mixture was stirred at 70°C for 1 h during which time the material dissolved.
The solution was cooled to room temperature, acidified with 1 N aqueous
hydrochloric acid and then extracted with 80% ethyl acetate/hexane. The
combined organic layers were dried over anhydrous MgSO4, filtered and
concentrated to afford a white solid. Recrystallization from
dichloromethane/hexane afforded the desired acid (4) as a white, crystalline
solid (42 mg, 91%): melting point 230°-231°C. The structure of the product
was also confirmed using IR, 1H NMR and mass spectroscopy.
brand name
Targretin (Ligand).
Therapeutic Function
Antineoplastic
General Description
Bexarotene is available in 75-mg capsules for oral administrationin the treatment of refractory cutaneous T-cell lymphoma.The agent is also available as a gel that may be usedtopically. The mechanism of action has not been fully establishedbut is thought to involve binding to retinoid receptorsresulting ultimately in the formation of transcription factorsthat promote cell differentiation and regulate cellular proliferation.168 Bexarotene has been demonstrated to activateapoptosis as a result of stimulation of caspase 3 and inhibitionof survivin, an antiapoptotic protein that would normallyinhibit caspase activity.Apoptosis is also stimulatedbecause of cleavage of poly(ADP-Ribose) polymerase,which is antiapoptotic. Reduced expression of the retinoidreceptor subtypes RXR and RAR has also been demonstratedfor the agent. Absorption is nearly complete afteroral administration and plasma protein binding is high(<99%).There is extensive metabolism in the liver togive 6- and 7-hydroxy-bexarotene and 6- and 7-oxobexaroteneas well as glucuronides of these metabolites andthe parent. Elimination occurs via the feces with an eliminationhalf-life of 7 hours. Adverse effects include hypercholesterolemia,hypertriglyceridemia, hypothyroidism, myelosuppression,nausea, and skin rash.
Biochem/physiol Actions
Bexarotene is a highly selective retinoid X receptor (RXR) agonist. It is an antineoplastic agent, already approved as an oral antineoplastic agent for cutaneous T cell lymphoma and being investigated against other cancers. A study has found that bexarotene in a mouse Alzheimer′s model lowered the most toxic form of β-amyloid peptide and increased cognitive ability. The activity in the mouse Alzheimer′s models are believed to be by activating PPARγ:RXR and LXR:RXR dimers which induces the expression of apoE and facilitates Aβ clearance and promotes microglial phagocytosis.
Pharmacokinetics
Bexarotene is a member of a subclass of retinoids that selectively activate retinoid X receptors (RXRs). These retinoid receptors have biologic activity distinct from that of retinoic acid receptors (RARs). Bexarotene is indicated for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma in patients who are refractory to at least one prior systemic therapy. Bexarotene selectively binds and activates retinoid X receptor subtypes (RXRα, RXRβ, RXRγ). RXRs can form heterodimers with various receptor partners such as retinoic acid receptors (RARs), vitamin D receptor, thyroid receptor, and peroxisome proliferator activator receptors (PPARs). Once activated, these receptors function as transcription factors that regulate the expression of genes that control cellular differentiation and proliferation. Bexarotene inhibits the growth in vitro of some tumor cell lines of hematopoietic and squamous cell origin. It also induces tumor regression in vivo in some animal models.
Pharmacology
Bexarotene is available in oral and topical formulations. Peak plasma levels are achieved within 2 hours of oral administration, although higher levels are obtained when the drug is ingested with a fatty meal. It is thought to be metabolized primarily by the hepatobiliary system, with a terminal half-life of approximately 7 hours. Topical and oral bexarotene are approved for earlystage (patch and plaque) cutaneous T-cell lymphoma that is refractory to at least one other therapy. Oral bexarotene is also approved for refractory cases of advanced disease; however, the best response has been noted in early disease. Local irritation, such as burning, pruritus, and irritant contact dermatitis, is common following topical application.
Clinical Use
Antineoplastic agent:
Treatment of skin manifestations of cutaneous T-cell
lymphoma
Side Effects
Major side effects seen after systemic administration include dyslipidemia, leukopenia, liver function test abnormalities, and possibly development of cataracts. Unlike other systemic retinoids, oral bexarotene causes thyroid abnormalities in approximately half of patients, which may necessitate treatment for hypothyroidism. Bexarotene is teratogenic and should not be prescribed in topical or oral form to women of childbearing potential unless a negative serum pregnancy test has been obtained and the patient agrees in writing to use two effective forms of contraception from 1 month before to 1 month after treatment.
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis)
Lipid-regulating drugs: concentration increased by
gemfibrozil - avoid.
Metabolism
Not Available
Metabolism
Hepatic metabolism. Studies suggest glucuronidation as a metabolic pathway, and that cytochrome P450 3A4 is the major cytochrome P450 isozyme responsible for formation of the oxidative metabolites. Bexarotene metabolites have little pharmacological activity. No studies have been done in renal failure although the pharmacokinetic data indicates that renal elimination is a minor excretory pathway
Storage
Store at +4°C
References
1) Boehm et al. (1995), Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells; J. Med. Chem., 38 3146 2) Gniadecki et al. (2007), The optimal use of bexarotene in cutaneous T-cell lymphoma; Br. J. Dermatol., 157 433 3) Bischoff et al. (1998), Beyond tamoxifen: the retinoid X receptor-selective ligand LGD1069 (TARGRETIN) causes complete regression of mammary carcinoma; Cancer Res., 58 479 4) Cramer et al. (2012), ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models; Science, 335 1503 5) Boehm-Cagan and Michaelson (2014), Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene; J. Neurosci., 34 7293
Properties of Bexarotene
| Melting point: | 230-231°C |
| Boiling point: | 489.7±44.0 °C(Predicted) |
| Density | 1.042 |
| RTECS | DH6834830 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO and ethanol |
| form | White powder |
| pka | 4.08±0.10(Predicted) |
| color | white to beige |
| λmax | 264nm(MeOH)(lit.) |
| Merck | 14,1194 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 week. |
Safety information for Bexarotene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Bexarotene
| InChIKey | NAVMQTYZDKMPEU-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

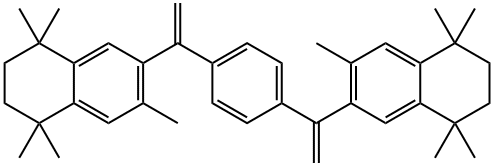
![Benzoic acid, 4-[1-(5,6,7,8-tetrahydro-3,5,5,8,8-pentamethyl-2-naphthalenyl)ethenyl]-, methyl ester](https://img.chemicalbook.in/CAS2/GIF/153559-48-9.gif)

You may like
-
 153559-49-0 Bexarotene 98%View Details
153559-49-0 Bexarotene 98%View Details
153559-49-0 -
 Bexarotene 98%View Details
Bexarotene 98%View Details
153559-49-0 -
 Bexarotene 153559-49-0 98%View Details
Bexarotene 153559-49-0 98%View Details
153559-49-0 -
 Bexarotene CAS 153559-49-0View Details
Bexarotene CAS 153559-49-0View Details
153559-49-0 -
 Bexarotene >98% (HPLC) CAS 153559-49-0View Details
Bexarotene >98% (HPLC) CAS 153559-49-0View Details
153559-49-0 -
 Bexarotene CAS 153559-49-0View Details
Bexarotene CAS 153559-49-0View Details
153559-49-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
