Silodosin
- CAS NO.:160970-54-7
- Empirical Formula: C25H32F3N3O4
- Molecular Weight: 495.53
- MDL number: MFCD00930170
- EINECS: 814-909-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-26 18:29:04
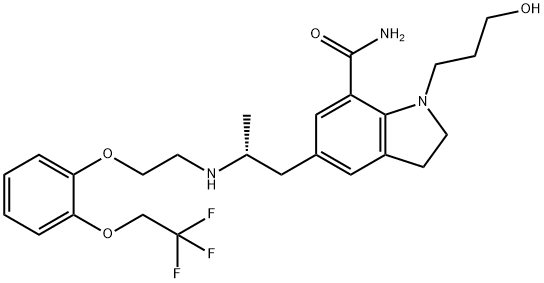
What is Silodosin?
Absorption
The absolute bioavailability is approximately 32%. Following oral administration of silodosin 8 mg once daily in healthy male subjects, Cmax was 61.6 ± 27.54 ng/mL and AUC was 373.4 ± 164.94 ng x hr/mL. The Tmax was 2.6 ± 0.90 hours. Silodosin glucuronide or KMD-3213G, the main metabolite of silodosin, has an AUC three- or four fold higher than for the parent compound.
A moderate fat or calorie meal reduces Cmax by 18% to 43% and AUC by 4% to 49%, as well as Tmax by about one hour. However, the US prescribing information recommends drug intake with meals to avoid the potential adverse effects associated with high plasma drug concentrations.
Toxicity
Oral LD50 is 800 mg/kg in rats.
In clinical trials, postural hypotension was the most common dose-limiting adverse event. In case of drug overdose leading to hypotension, the patient should be placed in a supine position to restore blood pressure and normalize heart rate. Further measures, such as administration of intravenous fluids, may be initiated. In case of the use of vasopressors, renal function should be monitored and supported as needed. Since silodosin is highly bound to plasma proteins, dialysis is unlikely to be beneficial.
Description
Silodosin, an a1A adrenoceptor (a1A-AR) antagonist selective for prostatic receptors, was launched as an oral treatment for dysuria associated with benign prostatic hypertrophy (BPH). The regulation of smooth muscle tone in the bladder neck and prostate is thought to be primarily mediated by a1A-AR. Blockade of these receptors can cause smooth muscle relaxation in these areas, resulting in improved symptoms and urinary flow rates. Conversely, a1B-AR are largely located on vascular smooth muscle, and antagonism of these receptors can cause tissue relaxation and potentially decrease cardiac compensation mechanisms involved in regulating blood pressure.
Chemical properties
White Solid
Originator
Kissei (Japan)
The Uses of Silodosin
Silodosin(Rapaflo) is an α1-adrenoceptor antagonist with high uroselectivity. It causes practically no orthostatic hypotension (in contrast to other α1 blockers). Since Silodosin is a highly selective inhibitor of the α1A adrenergic receptor, it causes pr
The Uses of Silodosin
An α1a-adrenoceptor antagonist. It is used in treatment of benign prostatic hypertophy.
The Uses of Silodosin
Silodosin is an α1a-adrenoceptor antagonist. It is used in treatment of benign prostatic hypertrophy.
What are the applications of Application
Silodosin is an α1-adrenoceptor antagonist with high uroselectivity
Indications
Silodosin is indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia (BPH). It is not indicated for the treatment of hypertension.
Background
Silodosin is a selective antagonist of alpha(α)-1 adrenergic receptors that binds to the α1A subtype with the highest affinity. α1-adrenergic receptors regulate smooth muscle tone in the bladder neck, prostate, and prostatic urethra: the α1A subtype accounts for approximately 75% of α1-adrenoceptors in the prostate.
Silodosin was first approved by the FDA in October 2008 and it is also approved in Europe and Canada. Silodosin is available as oral capsules with common trade names Rapaflo and Urorec. It is indicated for the symptomatic treatment of benign prostatic hyperplasia in adults. Most commonly affecting males over the age of 40 years, benign prostatic hyperplasia is the non-malignant enlargement of the prostate gland, associated with lower urinary tract symptoms that have a negative impact on the quality of life of patients. Silodosin works by binding to α1A-adrenoceptors with high affinity and relaxing the lower urinary tract, thereby improving urinary symptoms and alleviating bladder outlet obstruction.
Definition
ChEBI: Silodosin is an indolecarboxamide.
brand name
Urief
Pharmacokinetics
Silodosin is an antagonist of α1-adrenoceptors. It has the highest selectivity for the α1A-adrenoceptor subtype, with a 162-fold greater affinity than α1B-adrenoceptor and about a 50-fold greater affinity than for α1D-adrenoceptor. In clinical trials, silodosin improved maximum urinary flow rate, voiding symptoms, and storage symptoms of benign prostatic hyperplasia. Following oral administration, silodosin had a rapid onset of effect in men, with early effects of relieving lower urinary tract symptoms occurring within two to six hours post-dose.
Silodosin inhibited the human ether-a-go-go-related gene (HERG) tail current; however, it has weak cardiovascular effects. As with all α1-adrenoceptor antagonists blocking α1-adrenoceptors in the iris dilator muscle, silodosin may cause intraoperative floppy iris syndrome (IFIS), which is characterized by small pupils and iris billowing during cataract surgery in patients taking α1-AR antagonists.
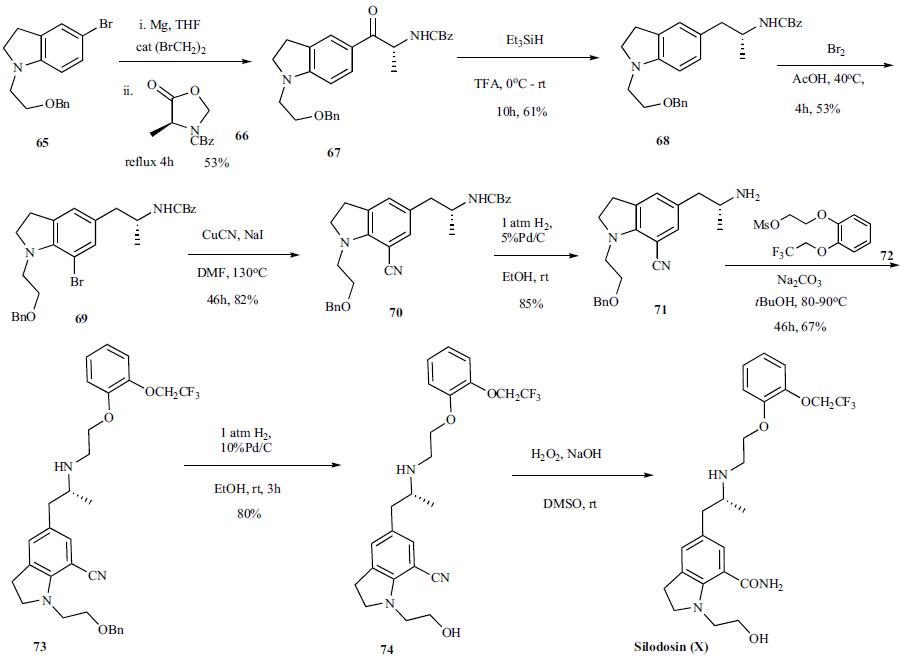
Synthesis
The synthesis of silodosin has been disclosed in several patents. The latest synthetic route disclosed in the 2006 patent is highlighted in the scheme. The synthesis started with Grignard generation from readily available bromoindoline 65 by treating it with Mg in the presence of a catalytic dibromoethane in THF. After initiation of the reaction with some heat and refluxing at a steady rate, CBZ protected oxazolidinone 66 [39b] was added over 1 h, refluxed for 4 h and then stirred at room temperature for 2 days. The reaction was quenched with 6 M aqueous HCl and stirred for 12 h after which time the reaction was worked up to provide product 67 in 53% yield. Ketone 67 was then treated with triethylsilane in TFA at 0oC and stirred at room temperature for 10 h to provide amine 68 in 61% yield. Bromination of the indoline 68 with bromine in warm acetic acid furnished bromide 69 in 53% yield which was reacted with copper cyanide in DMF at 130oC to give the cyano indoline 70 in 82% yield. Selective deprotection of the benzyloxycarbonyl over the benzyl group was accomplished by reacting indoline 70 with 1 atm hydrogen in the presence of 5% Pd/C in ethanol at room temperature. The resulting free amine 71 was then reacted with mesylate 72 in t-butanol with sodium carbonate as base at 80-90oC for 46 h to provide 73 in 67% yield. Removal of the benzyl ether was accomplished by reacting 73 with 1 atm hydrogen in the presence of 10%Pd/C to give alcohol 74, which upon hydrolysis provided the desired silodosin (X). No yield for the final reaction was given.
Metabolism
The main metabolite of silodosin is silodosin glucuronide (KMD-3213G), which is a pharmacologically active metabolite formed by direct glucuronide conjugation mediated by UDP-glucuronosyltransferase 2B7 (UGT2B7). Silodosin glucuronide reaches plasma exposure (AUC) approximately four times greater than that of silodosin. The second major metabolite, KMD-3293, is formed from dehydrogenation catalyzed by alcohol and aldehyde dehydrogenases. KMD-3293 has negligible pharmacological activity and reaches plasma exposures similar to that of silodosin. Silodosin is also metabolized by CYP3A4, which catalyzes the oxidation reaction.
Other than glucuronidation, dehydrogenation, and oxidation as its main metabolic pathways, silodosin can also undergo dealkylation (KMD-3289), N-dealkylation, hydroxylation, glucosylation, and sulfate conjugation. Metabolites of silodosin can undergo a series of further metabolic pathways.
Storage
Store at -20°C
Properties of Silodosin
| Melting point: | >104°C (dec.) |
| Boiling point: | 601.4±55.0 °C(Predicted) |
| alpha | D25 -14.0° (c = 1.01 in methanol) |
| Density | 1.249±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | Aqueous Base (Slightly, Heated Sonicated), DMSO (Slightly, Sonicated), Methanol |
| form | Solid |
| pka | 14.85±0.10(Predicted) |
| color | White to Off-White |
| CAS DataBase Reference | 160970-54-7(CAS DataBase Reference) |
Safety information for Silodosin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Silodosin
Silodosin manufacturer
Sumar biotech LLP
DR ACHARYA LABORATORIES PVT LTD
Kavya Pharma
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![5-[(2R)-2-Aminopropyl]-1-[3-(benzoyloxy)propyl]-2,3-dihydro-1H-indole-7-carbonitrile (2R,3R)-2,3-dihydroxybutanedioate](https://img.chemicalbook.in/CAS/20180808/GIF/239463-85-5.gif)
![2-[2-(2,2,2-Trifluoroethoxy)phenoxy]ethyl methanesulfonate](https://img.chemicalbook.in/CAS2/GIF/160969-03-9.gif)
![1H-Indole-7-carbonitrile, 2,3-dihydro-1-(3-hydroxypropyl)-5-[(2R)-2-[[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl]aMino]propyl]-](https://img.chemicalbook.in/CAS/GIF/885340-13-6.gif)
You may like
-
 Silodosin 97%View Details
Silodosin 97%View Details -
 Silodosin 99%View Details
Silodosin 99%View Details -
 Silodosin 160970-54-7 98%View Details
Silodosin 160970-54-7 98%View Details
160970-54-7 -
 Silodosin 98%View Details
Silodosin 98%View Details -
 Silodosin 98%View Details
Silodosin 98%View Details -
 SILODOSIN 95-99 %View Details
SILODOSIN 95-99 %View Details
160970-54-7 -
 Silodosin 98% CAS 160970-54-7View Details
Silodosin 98% CAS 160970-54-7View Details
160970-54-7 -
 Silodosin ip/bp/usp cas 160970-54-7View Details
Silodosin ip/bp/usp cas 160970-54-7View Details
160970-54-7
