Anidulafungin
- CAS NO.:166663-25-8
- Empirical Formula: C58H73N7O17
- Molecular Weight: 1140.24
- MDL number: MFCD00917070
- EINECS: 658-060-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
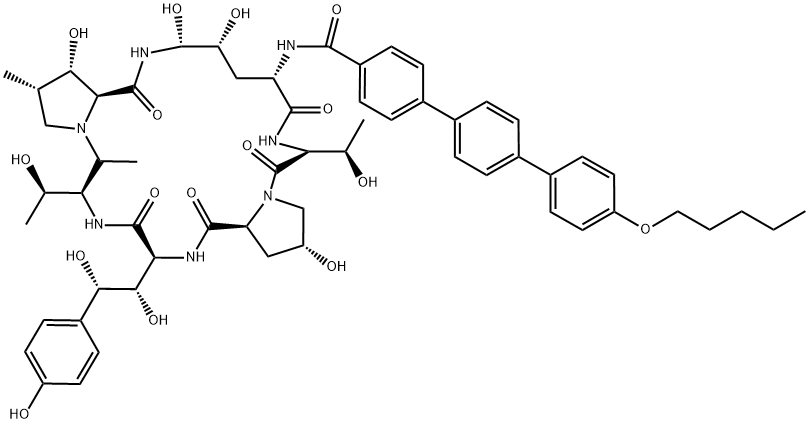
What is Anidulafungin?
Toxicity
During clinical trials a single 400 mg dose of anidulafungin was inadvertently administered as a loading dose. No clinical adverse events were reported. The maximum non-lethal dose of anidulafungin in rats was 50 mg/kg, a dose which is equivalent to 10 times the recommended daily dose for esophageal candidiasis (50mg/day).
Description
Anidulafungin, a semi-synthetic derivative of echinocandin B, has been developed and launched as an intravenous treatment for serious fungal infections, such as candidemia, Candida-derived peritonitis, intra-abdominal abscesses, and esophageal candidiasis. As a non-competitive inhibitor of 1,3-b-D-glucan synthase, which is responsible for the formation of glucan polymers, anidulafungin interferes with the cell wall synthesis of most pathogenic fungi. This mode of action is characteristic of the echinocandin class of antifungals. While the first member of this class, cilofungin, was withdrawn due to toxicity associated with the formulation vehicle, anidulafungin follows the successful introduction of caspofungin and micafungin. Compared to the other echinocandins, anidulafungin appears to be more potent (MIC90 ofr0.25 mg/mL for C.albicans, 0.5 mg/mL for C.glabrata, 1 mg/mL for C. krusel and C.tropicalis, 2mg/mL for C.lusitaniae, and 2 mg/mL for Aspergillus spp) and is devoid of significant drug interactions since it is neither an inhibitor nor substrate of the cytochrome P450 isoenzymes. The emergence of the echinocandins circumvents the concern regarding the rising resistance to the azole and amphotericin B antifungals; no cross-resistance is expected because the echinocandins work at the cell wall rather than the cell membrane.
Originator
Eli Lilly (US)
The Uses of Anidulafungin
Anidulafungin is a semi-synthetic cyclic lipopeptide belonging to the echinocandin class that was reported in 1995 and commercially developed by Eli Lilly. Anidulafungin inhibits the synthesis of β-(1,3)-D-glucan, an essential component of the cell wall of susceptible fungi and is extensively referenced in the literature with over 400 citations.
The Uses of Anidulafungin
nucleoside reverse transcriptase inhibitor (NRTI) for HIV treatment in adults
Indications
For use in the treatment of the following fungal infections: Candidemia and other forms of Candida infections (intra-abdominal abscess, and peritonitis), Aspergillus infections, and esophageal candidiasis. Also considered an alternative treatment for oropharyngeal canaidiasis.
What are the applications of Application
Anidulafungin is a semisynthetic echinocandin and antifungal
Background
Anidulafungin or Eraxis is an anti-fungal drug manufactured by Pfizer that gained approval by the Food and Drug Administration (FDA) in February 21, 2006; it was previously known as LY303366. There is preliminary evidence that it has a similar safety profile to caspofungin.
Definition
ChEBI: A semisynthetic echinocandin anti-fungal drug. It is active against Aspergillus and Candida species and is used for the treatment of invasive candidiasis.
brand name
Eraxis (Vicuron).
Antimicrobial activity
It is active against Aspergillus spp., Candida spp. and the cyst stage of Pneumocystis jirovecii. Resistance has not yet been reported.
Pharmaceutical Applications
A semisynthetic lipopeptide derived from a fermentation product of Aspergillus nidulans. Formulated for intravenous infusion.
Pharmacokinetics
Anidulafungin is a semi-synthetic lipopeptide synthesized from a fermentation product of Aspergillus nidulans. Anidulafungin is an echinocandin, a class of antifungal drugs that inhibits the synthesis of 1,3-β-D-glucan, an essential component of fungal cell walls. Anidulafungin is active in vitro against many Candida, as well as some Aspergillus. Like other echinocandins, anidulafungin is not active against Cryptococcus neoformans, Trichosporon, Fusarium, or zygomycetes.
Pharmacokinetics
Cmax 100 mg 1-h infusion: c. 9 mg/L end infusion
Plasma half-life: 18–27 h
Volume of distribution: 0.6 L/kg
Plasma protein binding: 84%
Blood concentrations increase in proportion to dosage. The
steady state is achieved on the first day after a loading dose
(twice the daily maintenance dose).
Distribution
Levels in the CSF are negligible.
Metabolism and excretion
Unlike caspofungin and micafungin, anidulafungin is not metabolized by the liver, but undergoes slow non-enzymatic degradation in the blood to a peptide breakdown product which is enzymatically degraded and excreted in the feces and bile. About 30% of a dose is eliminated in the feces, of which less than 10% is unchanged drug. Less than 1% of a dose is excreted in the urine. No dosage adjustment is required in patients with hepatic or renal impairment. Anidulafungin is not cleared by hemodialysis.
Clinical Use
Candidemia and certain invasive forms of candidosis
Esophageal candidosis
Side Effects
Occasional histamine-mediated infusion-related reactions, injection site reactions and transient abnormalities of liver enzymes have been reported.
Metabolism
Hepatic metabolism of anidulafungin has not been observed. Anidulafungin is not a clinically relevant substrate, inducer, or inhibitor of cytochrome P450 (CYP450) isoenzymes. Anidulafungin undergoes slow chemical degradation at physiologic temperature and pH to a ring-opened peptide that lacks antifungal activity.
Metabolism
Hepatic metabolism of anidulafungin has not been
observed. Anidulafungin is not a clinically relevant
substrate, inducer, or inhibitor of cytochrome P450
isoenzymes.
Anidulafungin undergoes slow chemical degradation at
physiologic temperature and pH to a ring-opened peptide
that lacks antifungal activity. The ring-opened product
is subsequently converted to peptidic degradants and
eliminated mainly through biliary excretion.
In a single-dose clinical study, radiolabelled
[14C]-anidulafungin (~88 mg) was administered to
healthy subjects. Approximately 30% of the administered
radioactive dose was eliminated in the faeces over 9 days,
of which less than 10% was intact drug. Less than 1%
of the administered radioactive dose was excreted in the
urine, indicating negligible renal clearance.
References
[1] zhanel gg1, karlowsky ja, harding ga, balko tv, zelenitsky sa, friesen m, kabani a, turik m, hoban dj. in vitro activity of a new semisynthetic echinocandin, ly-303366, against systemic isolates of candida species, cryptococcus neoformans, blastomyces dermatitidis, and aspergillus species. antimicrob agents chemother. 1997 apr;41(4):863-5.
Properties of Anidulafungin
| Melting point: | >196°C (subl.) |
| Boiling point: | 1477.0±65.0 °C(Predicted) |
| Density | 1.47±0.1 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly) |
| form | Solid |
| pka | 9.86±0.26(Predicted) |
| color | White to Pale Beige |
Safety information for Anidulafungin
Computed Descriptors for Anidulafungin
Anidulafungin manufacturer
New Products
Mercury(II) nitrate monohydrate, 98% 2-Chloro-5-nitrobenzoic acid, 98% 2-Benzylpyridine 2-Amino-2-methyl-1-propanol, 93% 3-Benzyloxybenzyl Alcohol Aluminum potassium sulfate dodecahydrate 2-Chloro Acetophenone Methyl-2-Methoxy-5-Sulfamoyl Benzoate Orthochlorobenzaldehyde (2-Chlorobenzaldehyde) Pyrrolidine Para Chloro Toluene (PCT) Diiodo Pentoxide 1-(Sulfamoylamino)Propane 1-phenyl-1,2,3,4-tetrahydroisoquinoline 2-amino benzyl alcohol 3,5-benzyloxy Acetophenone Veratric acid Pyridine-2-carboxaldehydeRelated products of tetrahydrofuran

You may like
-
 166663-25-8 Anidulafungin 98%View Details
166663-25-8 Anidulafungin 98%View Details
166663-25-8 -
 166663-25-8 98%View Details
166663-25-8 98%View Details
166663-25-8 -
 Anidulafungin 98%View Details
Anidulafungin 98%View Details
166663-25-8 -
 Anidulafungin 166663-25-8 98%View Details
Anidulafungin 166663-25-8 98%View Details
166663-25-8 -
 166663-25-8 98%View Details
166663-25-8 98%View Details
166663-25-8 -
 166663-25-8 Anidulafungin 98%View Details
166663-25-8 Anidulafungin 98%View Details
166663-25-8 -
 166663-25-8 98%View Details
166663-25-8 98%View Details
166663-25-8 -
 Anidulafungin 98%View Details
Anidulafungin 98%View Details
166663-25-8