VANADIUM (IV) CHLORIDE
Synonym(s):Vanadium tetrachloride
- CAS NO.:7632-51-1
- Empirical Formula: Cl4V
- Molecular Weight: 192.75
- MDL number: MFCD00061343
- EINECS: 231-561-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
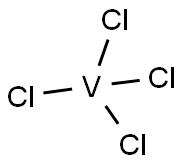
What is VANADIUM (IV) CHLORIDE?
Chemical properties
Red liquid. Decomposes slowly to vanadium trichloride and chlorine below63C. Soluble in absolute alcohol and ether. Nonflammable.
Chemical properties
Vanadium tetrachloride is a thick, reddishbrown liquid that gives off fumes on exposure to moist air.
The Uses of VANADIUM (IV) CHLORIDE
Preparation of vanadium trichloride, vanadium dichloride, and organovanadium compounds.
Air & Water Reactions
Forms acid mists in moist air. Reacts with water to form corrosive fumes of HCl. Handling Chemicals Safely 1980. p. 952).
Reactivity Profile
VANADIUM (IV) CHLORIDE decomposes by the action of light with the formation of Cl2 gas. Reacts as an acid to neutralize bases. These neutralizations generate heat. Usually does not react as either oxidizing agents or reducing agents but such behavior is not impossible. May catalyze organic reactions.
Health Hazard
CORROSIVE and/or TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire will produce irritating, corrosive and/or toxic gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
EXCEPT FOR ACETIC ANHYDRIDE (UN1715), THAT IS FLAMMABLE, some of these materials may burn, but none ignite readily. May ignite combustibles (wood, paper, oil, clothing, etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Flammable/toxic gases may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. Substance may be transported in a molten form.
Flammability and Explosibility
Not classified
Safety Profile
Poison by ingestion. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of VOx and Cl-. See also VANADIUM COMPOUNDS and HYDROCHLORIC ACID.
Potential Exposure
Vanadium tetrachloride is used as a fixative in textile dyeing and in the manufacture of other vanadium compounds.
Shipping
UN2444 Vanadium tetrachloride, Hazard class: 8; Labels: 8-Corrosive material.
Incompatibilities
Vanadium tetrachloride is chemically unstable, highly reactive with all forms of moisture, including humidity, releasing corrosive hydrogen chloride and/or chloride fumes. Keep away from light, UV, water, steam, lithium, chlorine, trifluoride, alcohols, organic, and combustible materials. Vanadium tetrachloride is a reactive chemical and is an explosion hazard. See storage and handling section. Corrosive to metals and may release flammable hydrogen gas. Vanadium tetrachloride decomposes by the action of light with the formation of Cl2 gas. Reacts as an acid to neutralize bases. These neutralizations generate heat. Usually does not react as either oxidizing agents or reducing agents but such behavior is not impossible. May catalyze organic reactions.
Properties of VANADIUM (IV) CHLORIDE
| Melting point: | −28 °C(lit.) |
| Boiling point: | 154 °C(lit.) |
| Density | 1.816 g/mL at 25 °C(lit.) |
| vapor pressure | 10.173hPa at 25℃ |
| Flash point: | 148.5°C/755mm |
| storage temp. | water-free area |
| solubility | diethyl ether: slightly soluble(lit.) |
| form | Liquid |
| color | Opaque reddish-brown |
| Water Solubility | soluble alcohol, ether [HAW93] |
| Sensitive | Moisture Sensitive |
| Dielectric constant | 3.1(Ambient) |
| EPA Substance Registry System | Vanadium chloride (VCl4), (T-4)- (7632-51-1) |
Safety information for VANADIUM (IV) CHLORIDE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for VANADIUM (IV) CHLORIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

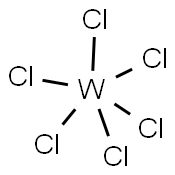
You may like
-
 VANADIUM(IV)CHLORIDE CAS 7632-51-1View Details
VANADIUM(IV)CHLORIDE CAS 7632-51-1View Details
7632-51-1 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1