7632-51-1
Product Name:
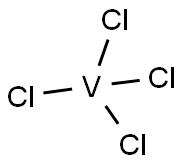
VANADIUM (IV) CHLORIDE
Formula:
Cl4V
Synonyms:
Vanadium tetrachloride
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Vanadium tetrachloride appears as a red liquid with a pungent odor. Boiling point 309.2 °F (154 °C). Corrosive to metals and tissue. |
|---|---|
| Color/Form | Red-brown, oily liquid |
| Odor | Pungent odor due to slow decomposition at room temperature with Cl2 formation |
| Boiling Point | 1518 °C |
| Melting Point | -28 °C |
| Solubility | Reacts with water |
| Density | 1.816 at 20 °C |
| Vapor Density | 6 (Air = 1) |
| Vapor Pressure | 7.63 [mmHg] |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | Decomposes by action of light to form chlorine gas. |
| Corrosivity | Corrosive to metals |
| Heat of Vaporization | 42.5 kJ/mol at 25 °C, 41.4 kJ/mol at 151 °C |
| Other Experimental Properties | Decomposes slowly to vanadium trichloride and chlorine below 63 °C |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 192.7 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 192.816418 g/mol |
| Monoisotopic Mass | 190.819368 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 19.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Vanadium tetrachloride appears as a red liquid with a pungent odor. Boiling point 309.2 °F (154 °C). Corrosive to metals and tissue.