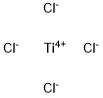
Titanous chloride
- CAS NO.:7705-07-9
- Empirical Formula: Cl2Ti
- Molecular Weight: 118.77
- MDL number: MFCD00011266
- EINECS: 231-728-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13

What is Titanous chloride?
Chemical properties
purple crystalline solid
Physical properties
Red-violet hexagonal crystals; hygroscopic; density 2.64 g/cm3; decomposes on heating above 425°C; also decomposes in water, evolving heat; soluble in alcohol, acetonitrile and certain amines; insoluble in hydrocarbons and ether.
The Uses of Titanous chloride
As powerful reducing agent, Titanous chlorideb can reduces nitrate to ammonia; when boiled with aqueous SO2, sulfur is separated; hence is used as an aqueous solution for estimation of nitro groups, ferric ions, per-salts, etc. Removes stains, etc. (stripper) in laundering.
The Uses of Titanous chloride
Titanium Chloride (≥12% HCl Solution) is ued in the optimized gas chromatography tandem mass spectrometry for 1,1''-sulfonylbis[2-(methylthio)ethane] quantification in human urine
Preparation
Titanium trichloride may be prepared by reducing titanium tetrachloride with hydrogen at 600°C. The tetrachloride may alternatively be reduced with aluminum, zinc, magnesium, tin, or by electrolysis.
General Description
A dark violet crystalline solid. Denser than water. Contact may burn skin, eyes, and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Used to make other chemicals.
Air & Water Reactions
Pyrophoric, very reactive with water and moisture in air produces hydrochloric acid, [Merck 11th ed. 1989]. Ignites spontaneously on contact with air; decomposed by water and water vapor forming HCl. [Handling Chemcials Safely 1980. p. 905].
Reactivity Profile
Acidic salts, such as TITANIUM TRICHLORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. Ethylene can polymerize at low pressure if catalyzed by titanium halides. (Sundaram, K. M, M. M. Shreehan, E. F. Olszewski. thylene. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2001.)
Hazard
Fire risk in the presence of oxidizing materials. Irritant to skin and tissue; open container only in oxygen-free or inert atmosphere.
Health Hazard
Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Fire Hazard
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.
Flammability and Explosibility
Pyrophoric
Safety Profile
A corrosive irritant to skin, eyes, and mucous membranes. A severe corrosive because it liberates heat and hydrochloric acid upon contact with moisture. If spilled on slun, wipe off with dry cloth before applying water. May ignite spontaneously in air. Flammable when exposed to heat or flame. Reacts violently with K, HF. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of Cl-. See also TITANIUM COMPOUNDS.
Purification Methods
It is a brown purple powder that is very reactive to H2O and pyrophoric when dry. It should be manipulated in a dry box. It is soluble in CH2Cl2 and tetrahydrofuran, and is used as a M solution in these solvents in the ratio of 2:1, and stored under N2. It is a powerful reducing agent. [Ingraham et al. Inorg Synth VI 52 1960.]
Properties of Titanous chloride
| Melting point: | 440 °C (dec.)(lit.) |
| Boiling point: | 100 °C |
| Density | 1.192 g/mL at 25 °C |
| solubility | reacts with H2O |
| form | powder |
| color | Purple |
| Water Solubility | Soluble |
| Sensitive | Air Sensitive |
| Merck | 14,9481 |
| Stability: | Stable, but reacts violently with water and is spontaneously flammable in air. |
| CAS DataBase Reference | 7705-07-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Titanium(iii) chloride(7705-07-9) |
| EPA Substance Registry System | Titanium chloride (TiCl3) (7705-07-9) |
Safety information for Titanous chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H290:Corrosive to Metals H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Titanous chloride
| InChIKey | ZWYDDDAMNQQZHD-UHFFFAOYSA-L |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


![2-[[(2-ethylphenyl)(2-hydroxyethyl)amino]methyl]-3,3-difluoro-Propanenitrile](https://img.chemicalbook.in/CAS/GIF/2647-14-5.gif)

You may like
-
 Titanium trichloride 98%View Details
Titanium trichloride 98%View Details -
 7705-07-9 TITANIUM TRICHLORIDE 50% SOLUTION AR,ACS 50.00%View Details
7705-07-9 TITANIUM TRICHLORIDE 50% SOLUTION AR,ACS 50.00%View Details
7705-07-9 -
 TITANIUM TRICHLORIDE 30% SOLUTION AR,ACS 7705-07-9 30.00%View Details
TITANIUM TRICHLORIDE 30% SOLUTION AR,ACS 7705-07-9 30.00%View Details
7705-07-9 -
 Titanium trichloride, solution ≥12% TiCl3 basis CAS 7705-07-9View Details
Titanium trichloride, solution ≥12% TiCl3 basis CAS 7705-07-9View Details
7705-07-9 -
 Titanium(III) Chloride CAS 7705-07-9View Details
Titanium(III) Chloride CAS 7705-07-9View Details
7705-07-9 -
 Titanium(III) chloride solution CAS 7705-07-9View Details
Titanium(III) chloride solution CAS 7705-07-9View Details
7705-07-9 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
