GERMANIUM(IV) CHLORIDE
Synonym(s):Germanium tetrachloride;Germanium(IV) chloride
- CAS NO.:10038-98-9
- Empirical Formula: Cl4Ge
- Molecular Weight: 214.45
- MDL number: MFCD00011029
- EINECS: 233-116-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
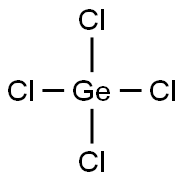
What is GERMANIUM(IV) CHLORIDE?
Chemical properties
Colorless liquid. decomposes in water. Insoluble in concentrated hydrochloric acid; soluble in carbon disulfide, chloroform, benzene, alcohol, and ether.
Physical properties
Colorless liquid; density 1.879 g/cm3 at 20°C and 1.844 g/cm3 at 30°C; refractive index 1.464; boils at 86.5°C; solidifies at -49.5°C; decomposes in water; soluble in alcohol, ether, benzene, chloroform and carbon tetrachloride; insoluble in concentrated hydrochloric and sulfuric acids.
The Uses of GERMANIUM(IV) CHLORIDE
Germanium(IV) chloride is used in the microwave preparation of Ge2Cl6, a colorless crystalline material which was included in a further study of low-valent germanium compounds.1
Germanium(IV) chloride is used in the preparation of many germanium compounds.
The Uses of GERMANIUM(IV) CHLORIDE
It is used as a catalyst in the conversion of carbohydrates into 5-hydroxymethylfurfural in ionic liquids, as a reducing agent in combination with triphenylphosphine (TPP) for the reduction of alpha-bromo carboxylic acid derivatives, and in the production of pure germanium. It also finds use as an intermediate for several optical processes. It is one of the most important dopants in silica glass for optical fibers. It is employed in the preparation of germanium dioxide which is used for wide camera lens, microscopy, IR-transparent glasses/windows/lenses, and for the core of fiber-optic lines.
What are the applications of Application
Germanium(IV) chloride is a precursor to Ge2-Cl6
Hazard
Toxic material
Flammability and Explosibility
Non flammable
Safety Profile
Poison by intravenous route. Mddly toxic by inhalation. A skin, severe eye, and mucous membrane irritant. Will react violently with water or steam to produce toxic and corrosive fumes. When heated to decomposition it emits toxic fumes of Cl-. See also GERMANIUM COMPOUNDS.
Purification Methods
Traces of Cl2 and HCl can be removed from the liquid by blowing dry air through it for a few hours at room temperature or by shaking it with Hg or Hg2Cl2 and then fractionating it in a vacuum. It decomposes on heating at 950o. It has a sharp penetrating odour and fumes in moist air to give a chalky coat of GeO2. It is slowly hydrolysed by H2O to give GeO2, but distils from conc HCl. [Foster et al. Inorg Synth II 109 1946, Dennis & Hance J Am Chem Soc 44 304 1922, Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 715 1963.] LACHRYMATORY. Glass powder (100-300 mesh). Washed with 10% HNO3, water and dry in air.
Properties of GERMANIUM(IV) CHLORIDE
| Melting point: | -49.5 °C |
| Boiling point: | 83 °C(lit.) |
| Density | 1.844 g/mL at 25 °C |
| vapor pressure | 76 mm Hg ( 20 °C) |
| refractive index | 1.4644 |
| Flash point: | 83.1°C |
| storage temp. | Store below +30°C. |
| solubility | Soluble in ether, benzene, chloroform, carbon tetrachloride. Insoluble in hydrochloric acid and sulphuric acid. |
| form | Liquid |
| Specific Gravity | 1.88 |
| color | Colorless |
| PH | <1 (H2O, 20℃) |
| Water Solubility | DECOMPOSES |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Merck | 14,4410 |
| Dielectric constant | 2.4(25℃) |
| CAS DataBase Reference | 10038-98-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Germanium tetrachloride(10038-98-9) |
| EPA Substance Registry System | Germane, tetrachloro- (10038-98-9) |
Safety information for GERMANIUM(IV) CHLORIDE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H290:Corrosive to Metals H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P234:Keep only in original container. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for GERMANIUM(IV) CHLORIDE
GERMANIUM(IV) CHLORIDE manufacturer
JSK Chemicals
ALS India Life Sciences Pvt. Ltd
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
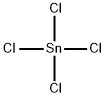


You may like
-
 Germanium(IV) chloride CAS 10038-98-9View Details
Germanium(IV) chloride CAS 10038-98-9View Details
10038-98-9 -
 Germanium(IV) chloride CAS 10038-98-9View Details
Germanium(IV) chloride CAS 10038-98-9View Details
10038-98-9 -
 Germanium(IV) chloride CAS 10038-98-9View Details
Germanium(IV) chloride CAS 10038-98-9View Details
10038-98-9 -
 Germanium(IV) chloride CAS 10038-98-9View Details
Germanium(IV) chloride CAS 10038-98-9View Details
10038-98-9 -
 10038-98-9 Germanium(IV) chloride, 95% 99%View Details
10038-98-9 Germanium(IV) chloride, 95% 99%View Details
10038-98-9 -
 Germanium(IV) chloride, 99.9999% CAS 10038-98-9View Details
Germanium(IV) chloride, 99.9999% CAS 10038-98-9View Details
10038-98-9 -
 Germanium(IV) chloride CAS 10038-98-9View Details
Germanium(IV) chloride CAS 10038-98-9View Details
10038-98-9 -
 Germanium(IV) chloride 10038-98-9 95.00%View Details
Germanium(IV) chloride 10038-98-9 95.00%View Details
10038-98-9
