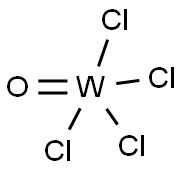
Tungsten(VI) Chloride
Synonym(s):Tungsten hexachloride
- CAS NO.:13283-01-7
- Empirical Formula: Cl6W
- Molecular Weight: 396.56
- MDL number: MFCD00011463
- EINECS: 236-293-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-16 09:55:30
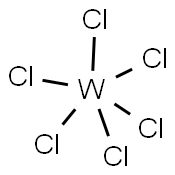
What is Tungsten(VI) Chloride?
Description
Tungsten(VI) chloride, WCl6, is a dark violet blue species that exists as a volatile solid under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. As a d0 ion, W(VI) forms diamagnetic derivatives. WCl6 is octahedral with equivalent W-Cl distances of 2.24-2.26 ? .
Chemical properties
dark grey-violet crystalline powder. Tungsten(VI) chloride is a blue-black solid at room temperature. At lower temperatures, it turns into wine red. A red form of the compound can be formed by quickly condensing its vapor, which transforms to the blue-black form on gentle heating. It is easily hydrolyzed, even by moist air, giving the orange oxychlorides WOCl4 and WO2Cl2, and then, tungsten trioxide. WCl6 is soluble in carbon disulfide, carbon tetrachloride, and phosphorus oxychloride. Methylation with trimethylaluminum forms hexamethyl tungsten.
The Uses of Tungsten(VI) Chloride
Precursor to tungsten-based carbenes which catalyze the olefin metathesis reactions of 1- and 2-octene.
The Uses of Tungsten(VI) Chloride
A precursor to tungsten based carbenes.
The Uses of Tungsten(VI) Chloride
It is a precursor for many tungsten compounds. It is used in tungsten plating, purification of tungsten, and olefin polymerization. It is also used as petroleum catalyst, and in intelligent glass. It can be used for deoxygenation of epoxides and as a reagent in organic synthesis.
What are the applications of Application
Tungsten(VI) chloride is a precursor to tungsten based carbenes
Definition
ChEBI: Tungsten hexachloride is a tungsten coordination entity.
Preparation
WCl6 can be prepared by chlorinating
tungsten metal in a sealed tube at 600℃.
W +3Cl2→WCl6
Flammability and Explosibility
Not classified
Purification Methods
Sublime it in a stream of Cl2 in a high temperature furnace and collect it in a receiver cooled in a Dry Ice-acetone bath in an inert atmosphere because it is sensitive to moisture. It is soluble in CS2, CCl4, CHCl3, POCl3, *C6H6, pet ether and Me2CO. Its solutions decompose on standing. Good crystals can be obtained by heating WCl6 in CCl4 to 100o in a sealed tube, followed by slow cooling (tablets of four-sided prisms). Store it in a desiccator over H2SO4 in the dark. [Leitzke & Holt Inorg Synth III 163 1950, Parterfield & Tyree Inorg Synth IX 133 1967, Hein & Herzog in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1420 1965.]
Properties of Tungsten(VI) Chloride
| Melting point: | 275 °C(lit.) |
| Boiling point: | 347 °C(lit.) |
| Density | 3.52 g/mL at 25 °C(lit.) |
| vapor pressure | 43 mm Hg ( 215 °C) |
| Flash point: | 346°C |
| solubility | Soluble in carbon disulfide, carbon tetrachloride and phosphorus oxychloride. |
| form | powder |
| color | Gray to dark gray-blue |
| Water Solubility | soluble organic solvents such as ethanol [HAW93] |
| Sensitive | Moisture Sensitive |
| Exposure limits | ACGIH: TWA 3 mg/m3 NIOSH: TWA 5 mg/m3; TWA 1 mg/m3; STEL 10 mg/m3; STEL 3 mg/m3 |
| CAS DataBase Reference | 13283-01-7(CAS DataBase Reference) |
| EPA Substance Registry System | Tungsten chloride (WCl6), (OC-6-11)- (13283-01-7) |
Safety information for Tungsten(VI) Chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tungsten(VI) Chloride
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Tungsten(VI) Chloride CAS 13283-01-7View Details
Tungsten(VI) Chloride CAS 13283-01-7View Details
13283-01-7 -
 Tungsten(VI) Chloride CAS 13283-01-7View Details
Tungsten(VI) Chloride CAS 13283-01-7View Details
13283-01-7 -
 Tungsten(VI) Chloride CAS 13283-01-7View Details
Tungsten(VI) Chloride CAS 13283-01-7View Details
13283-01-7 -
 Tungsten(VI) Chloride CAS 13283-01-7View Details
Tungsten(VI) Chloride CAS 13283-01-7View Details
13283-01-7 -
 Tungsten(VI) chloride CAS 13283-01-7View Details
Tungsten(VI) chloride CAS 13283-01-7View Details
13283-01-7 -
 Tungsten(VI) chloride CAS 13283-01-7View Details
Tungsten(VI) chloride CAS 13283-01-7View Details
13283-01-7 -
 Tungsten(VI) chloride CAS 13283-01-7View Details
Tungsten(VI) chloride CAS 13283-01-7View Details
13283-01-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
