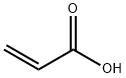
Urocanic acid
Synonym(s):3-(4-Imidazolyl)acrylic acid;Urocanic acid
- CAS NO.:104-98-3
- Empirical Formula: C6H6N2O2
- Molecular Weight: 138.12
- MDL number: MFCD00005203
- EINECS: 203-258-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
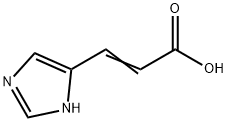
What is Urocanic acid?
Chemical properties
White to beige fine powder.
The Uses of Urocanic acid
Urocanic Acid is a biomarker in the fecal metabolic profiling of breast cancer patients.
Definition
ChEBI: Urocanic acid is an alpha,beta-unsaturated monocarboxylic acid that is prop-2-enoic acid substituted by a 1H-imidazol-4-yl group at position 3. It is a metabolite of hidtidine. It has a role as a chromophore and a human metabolite. It is an alpha,beta-unsaturated monocarboxylic acid and a member of imidazoles. It is a conjugate acid of a urocanate.
Biological Functions
Urocanic acid, produced in the upper layers of mammalian skin, is a major absorber of ultraviolet radiation (UVR). Urocanic acid (UCA) is formed in the upper layers of the epidermis where filaggrin, a histidine-rich filamentous protein produced after caspase-14 cleavage of profilaggrin, is broken down by proteinases into component amino acids.
General Description
4-Imidazoleacrylic acid also known as urocanic acid is a natural metabolite derived from histidine. It is majorly used as a UV chromophore with a strong absorption spectrum in the UV-B region in the range of 300-280 nm.
Synthesis
A method of producing trans-urocanic acid, which involves treating D-glucose with aqueous ammonia and formalin in molar ratio D-glucose: aqueous ammonia: formalin equal to 1:24:2.7, in water in the presence of basic copper carbonate at temperature 85-90°C for 3 hours to form (1S,2S,3S)-1-(1H-imidazol-4-yl)-butane-1,2,3,4-tetrazole, which is extracted in form of a hydrochloride. Through periodate splitting in water, the obtained (1S,2S,3S)-1-(1H-imidazol-4-yl)-butane-1,2,3,4-tetrazole is converted at room temperature to 1H-imidazole-4-carbaldehyde, condensation of which, in acetic anhydride in the presence of anhydrous potassium acetate at temperature 120°C for 2 hours, leads to formation of trans-urocanic acid with subsequent extraction thereof from the reaction mass.
Purification Methods
Crystallise the acid from water and dry it at 100o. The trans-isomer [3465-72-3] has m 225o (229-230o, 230-231o or 231o(dec, from H2O) and pK1 3.5 and pK2 5.6, and the picrate has m 225o(dec, from H2O). The cis-isomer [7699-35-6] has m 175-176o (178-179o or 180-184o dec, from H2O) and pK1 3.0 and pK2 6.7, and the picrate has m 204o (from H2O). [Beilstein 25 H 124, 25 I 536, 25 II 121, 25 III/IV 786.]
Properties of Urocanic acid
| Melting point: | 226-228 °C(lit.) |
| Boiling point: | 253.51°C (rough estimate) |
| Density | 1.3471 (rough estimate) |
| refractive index | 1.5100 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | 1.5g/l |
| form | Powder |
| pka | 2.94±0.10(Predicted) |
| color | White to beige |
| Water Solubility | SLIGHTLY SOLUBLE |
| BRN | 81405 |
| InChI | InChI=1S/C6H6N2O2/c9-6(10)2-1-5-3-7-4-8-5/h1-4H,(H,7,8)(H,9,10) |
| CAS DataBase Reference | 104-98-3(CAS DataBase Reference) |
| EPA Substance Registry System | 2-Propenoic acid, 3-(1H-imidazol-4-yl)- (104-98-3) |
Safety information for Urocanic acid
Computed Descriptors for Urocanic acid
| InChIKey | LOIYMIARKYCTBW-UHFFFAOYSA-N |
| SMILES | C(O)(=O)C=CC1NC=NC=1 |
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran




You may like
-
 Urocanic acid 95% CAS 104-98-3View Details
Urocanic acid 95% CAS 104-98-3View Details
104-98-3 -
 4-Imidazoleacrylic acid CAS 104-98-3View Details
4-Imidazoleacrylic acid CAS 104-98-3View Details
104-98-3 -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
