Trimesic acid
Synonym(s):BTC;TMA;Trimesic acid;H3BTC;1,3,5-Benzenetricarboxylic acid
- CAS NO.:554-95-0
- Empirical Formula: C9H6O6
- Molecular Weight: 210.14
- MDL number: MFCD00002517
- EINECS: 209-077-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:58
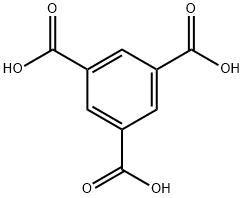
What is Trimesic acid?
Chemical properties
off-white powder
The Uses of Trimesic acid
Trimesic acid is mainly used in the industry of poxy curing agent ,Adhesives and coating material ,Engineering plastic ,Synthetic fiber ,Cross-linking agent for alkyd resin ,Plasticizer ,Pharmaceutical intermediates, Desalination membrane etc.
The Uses of Trimesic acid
Trimesinic Acid is used for multifunctional therapeutics. It shows inhibitory activity against Torpedo Californica acetylcholinesterase and prevented amyloid-β peptide aggregate formation.
What are the applications of Application
Trimesic acid is a planar benzoic acid with antiviral and chemical transport properties
Definition
ChEBI: Trimesic acid is a tricarboxylic acid that consists of benzene substituted by carboxy groups at positions 1, 3 and 5. It is a tricarboxylic acid and a member of benzoic acids. It is a conjugate acid of a benzene-1,3,5-tricarboxylate(1-).
General Description
Trimesic acid (TMA), also known as 1,3,5-benzenetricarboxylic acid, has a self-organized 2D molecular network structure formed by intermolecular hydrogen bonds between the carboxylic acid residues in bulk crystals. TMA can be used in the synthesis of metal-organic frameworks (MOFs), which can be used as organic linkers potentially utilized in sensors.
Flammability and Explosibility
Not classified
Synthesis
Trimesic acid can be produced by Friedel – Crafts methylation of toluene or xylene with chloromethane in the presence of aluminum chloride.It is also formed by liquid-phase disproportionation of xylene in the presence of aluminum chloride.These reactions can be carried out in the gas phase at high temperature on aluminum silicate catalysts.
Purification Methods
Crystallise the acid from water. The trimethyl ester has m 144o (from MeOH or MeOH/H2O). [Beilstein 9 H 978, 9 IV 3747.]
Properties of Trimesic acid
| Melting point: | >300 °C(lit.) |
| Boiling point: | 309.65°C (rough estimate) |
| Density | 1.4844 (rough estimate) |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1.6000 (estimate) |
| Flash point: | 328°C |
| storage temp. | Store below +30°C. |
| solubility | 26.3g/l |
| form | Powder |
| pka | pK1: 2.12;pK2: 4.10;pK3: 5.18 (25°C) |
| color | white |
| Water Solubility | Soluble in water, ethanol and methanol. |
| BRN | 2053080 |
| Stability: | Stable, but may be light sensitive. |
| CAS DataBase Reference | 554-95-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,3,5-Benzenetricarboxylic acid(554-95-0) |
| EPA Substance Registry System | 1,3,5-Benzenetricarboxylic acid (554-95-0) |
Safety information for Trimesic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Trimesic acid
| InChIKey | QMKYBPDZANOJGF-UHFFFAOYSA-N |
Trimesic acid manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 554-95-0 99%View Details
554-95-0 99%View Details
554-95-0 -
 Benzene-1,3,5-tricarboxylic acid, 99% CAS 554-95-0View Details
Benzene-1,3,5-tricarboxylic acid, 99% CAS 554-95-0View Details
554-95-0 -
 1,3,5-Benzenetricarboxylic Acid CAS 554-95-0View Details
1,3,5-Benzenetricarboxylic Acid CAS 554-95-0View Details
554-95-0 -
 Benzene-1,3,5-tricarboxylic acid 98% CAS 554-95-0View Details
Benzene-1,3,5-tricarboxylic acid 98% CAS 554-95-0View Details
554-95-0 -
 Trimesic acid CAS 554-95-0View Details
Trimesic acid CAS 554-95-0View Details
554-95-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
