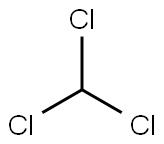
Chloral
- CAS NO.:75-87-6
- Empirical Formula: C2HCl3O
- Molecular Weight: 147.39
- MDL number: MFCD00036214
- EINECS: 200-911-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
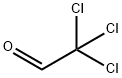
What is Chloral?
Description
Chloral is a combustible, oily liquid with a pungent irritating odor. Molecular weight=147.38; Boilingpoint=97-98℃; Flash point=75℃. Hazard Identification(based on NFPA 704 M Rating System): Health 3,Flammability 2, Reactivity 1. Soluble in water. Chloralhydrate is colorless crystals, with characteristic odor.Molecular weight=165.40; Boiling point=97℃ (decomposes); Freezing/Melting point=57-60℃. Hazard Identification (based on NFPA 704 M Rating System): Health 3,Flammability 2, Reactivity 1. Very soluble in water.
Chemical properties
colourless oily liquid with a pungent odour
Chemical properties
Chloral is a combustible, oily liquid with a pungent irritating odor.
The Uses of Chloral
manufacture of chloral hydrate, DDT.
The Uses of Chloral
Chloral is used in medicine as a hypnotic.
Definition
A colorless liquid aldehyde made by chlorinating ethanal. It was used to make the insecticide DDT. It can be hydrolyzed to give 2,2,2- trichloroethanediol (chloral hydrate, CCl3CH(OH)2). Most compounds with two –OH groups on the same carbon atom are unstable. However, in this case the effect of the three chlorine atoms stabilizes the compound. It is used as a sedative.
Production Methods
Chloral can be prepared by action of Cl2 on ethanol, chlorination of acetaldehyde, oxidation of 1,1,2-trichloroethylene in the presence of a catalyst (FeCl3, AlCl3, TiCl4 or SbCl3, and by reaction of CCl4 with formaldehyde.
General Description
A colorless oily liquid with a penetrating odor. Reacts with water and denser than water. Contact may irritate skin, eyes and mucous membranes. Toxic by ingestion and inhalation. Used to make pesticides.
Air & Water Reactions
Chloral is sensitive to exposure to moisture and light. Soluble in water. Chloral reacts with water to form chloral hydrate.
Reactivity Profile
Chloral reacts with water to form chloral hydrate. Chloral polymerizes under the influence of light and in the presence of sulfuric acid forming a white solid trimer called metachloral.
Hazard
Toxic by ingestion. Probable carcinogen.
Health Hazard
INHALATION: Sore throat, shortness of breath, drowsiness, irritation of respiratory tract, unconsciousness. EYES: Redness, pain and blurred vision. SKIN: Redness and pain. INGESTION: Dizziness, drowsiness, nausea, and unconsciousness. Acute hazard: Poison may be fatal if inhaled, swallowed, or absorbed through skin.
Safety Profile
A poison. Mutation E data reported.
Potential Exposure
Chloral is used as an intermediate in the manufacture of such pesticides as DDT, methoxychlor, DDVP, naled, trichlorfon, and TCA. Chloral is also used in the production of chloral hydrate; used as a therapeutic agent with hypnotic, sedative, and narcotic effects; used in a time prior to the introduction of barbiturates
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention.Give large quantities of water and induce vomiting. Do notmake an unconscious person vomit.
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with chloralyou should be trained on its proper handling and storage.Protect from light, moisture, air, and acids. DEA regulationsrequire storage in a locked storage area. Store in tightlyclosed containers in a cool, well-ventilated area. Metal containers involving the transfer of this chemical should begrounded and bonded. Drums must be equipped with selfclosing valves, pressure vacuum bungs, and flame arresters.Use only nonsparking tools and equipment, especially whenopening and closing containers of this chemical. Sources ofignition, such as smoking and open flames, are prohibitedwhere this chemical is used, handled, or stored in a mannerthat could create a potential fire or explosion hazard.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Distil chloral, then dry it by distilling through a heated column of CaSO4. [Beilstein 1 H 616, 1 I 328, 1 II 467, 1 III 2663, 1 IV 3142 for anhydr, 1 IV 3143 for hydrate.]
Incompatibilities
Chloral hydrate reacts with strong bases forming chloroform. Contact with acids, or exposure to light may cause polymerization. Reacts with water, forming chloral hydrate. Reacts with oxidizers, with a risk of fire or explosions.
Waste Disposal
Incineration after mixing with another combustible fuel; care must be taken to assure complete combustion to prevent phosgene formation; an acid scrubber is necessary to remove the halo acids produced.
Properties of Chloral
| Melting point: | -57.5°C |
| Boiling point: | 94-98 °C |
| Density | 1.51 g/mL at 20 °C(lit.) |
| refractive index | n |
| Flash point: | 75°C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Sparingly), DMSO (Sparingly), Ethyl Acetate (Slightly) |
| form | Solid |
| pka | 10.04(at 25℃) |
| color | Off-White |
| Water Solubility | Soluble |
| Merck | 13,9699 |
| Dielectric constant | 6.7(Ambient) |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 75-87-6(CAS DataBase Reference) |
| IARC | 2A (Vol. 63, 84, 106) 2014 |
| NIST Chemistry Reference | Acetaldehyde, trichloro-(75-87-6) |
| EPA Substance Registry System | Chloral (75-87-6) |
Safety information for Chloral
Computed Descriptors for Chloral
Chloral manufacturer
CEFA CILINAS BIOTICS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 75-87-6 99%View Details
75-87-6 99%View Details
75-87-6 -
 75-87-6 Chloral 99.00%View Details
75-87-6 Chloral 99.00%View Details
75-87-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
