Triclosan
Synonym(s):5-Chloro-2-(2,4-dichlorophenoxy)phenol;Irgasan;Triclosan;Triclosan - CAS 3380-34-5 - Calbiochem
- CAS NO.:3380-34-5
- Empirical Formula: C12H7Cl3O2
- Molecular Weight: 289.54
- MDL number: MFCD00800992
- EINECS: 222-182-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
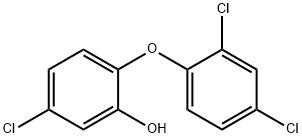
What is Triclosan?
Absorption
A study conducted in 2000 demonstrated that low amounts of triclosan can be absorbed through skin and can enter the bloodstream. [PMID: 10722890] Triclosan is rapidly absorbed and distributed in the human body (Sandborgh-Englund et al., 2006). Maximum concentrations are reached within three hours after oral intake. However, the metabolism and excretion of the compound is fast.
Toxicity
Oral LD50, Rat: 3700 mg/kg; Dermal LD50, Rabbit: 9300 mg/kg
Description
Triclosan is a broad-spectrum antibacterial agent that inhibits bacterial fatty acid synthesis. It is effective against Gram-negative and Gram-positive bacteria, as well as against Mycobacteria. Triclosan is used in a variety of products, including antiseptic soaps, deodorants, and hand washes.
Chemical properties
Solid
Physical properties
Triclosan is a slightly aromatic high-purity white crystalline powder; Solubility: slightly soluble in water, moderately soluble in dilute alkali, has high solubility in many organic solvents, in water-soluble solvents or surfactants After dissolving, it can be made into a transparent concentrated liquid product.
Originator
Anti-Bac,Bentfield Europe BV,Netherlands
The Uses of Triclosan
triclosan is a preservative considered to have a low sensitizing potential in leave-on preparations.
The Uses of Triclosan
Used as bacteriostat and preservative for cosmetic and detergent compositions. Antiseptic, disinfectant.
The Uses of Triclosan
anticonvulsant
The Uses of Triclosan
Bacteriostat and preservative for cosmetic and detergent preparations.
What are the applications of Application
Triclosan is an inhibitor of fatty acid synthase
Background
Triclosan is an aromatic ether that is phenol, which is substituted at C-5 by a chloro group and at C-2 by a 2,4-dichlorophenoxy group. It is used in various common household products, including soaps, mouthwashes, dish detergents, toothpaste, deodorants, and hand sanitizers. It is also used for surgical scrubs and personnel hand washes in healthcare settings.
Definition
ChEBI: An aromatic ether that is phenol which is substituted at C-5 by a chloro group and at C-2 by a 2,4-dichlorophenoxy group. It is widely used as a preservative and antimicrobial agent in personal care products such as soaps, skin creams, toothpaste and deodo ants as well as in household items such as plastic chopping boards, sports equipment and shoes.
Indications
Triclosan is a broad-spectrum antimicrobial compound. It was originally used in soaps, antiperspirants, and cosmetic toiletries as a germicide. Today, triclosan is incorporated into toothpaste because of its wide spectrum of antimicrobial activities and low toxicity.
Manufacturing Process
476 g of 4-chloro-2-methoxyphenol(4-chloroguaiacol) and 578 parts of 3,4-
dichloro-1-nitrobenzene are melted in 400 ml of diethylene glycoldimethyl
ether in a three necked flask fitted with a stirrer and sloping condenser and,
at about 120°C, 342 g of 49.6% potassium hydroxide solution are added
drop-wise within about 4 h. The inner temperature is kept for 12 h at 140°-
150°C whereby water and slight amounts of organic substances distill off, as
partly occured during the dropwise addition of the potassium hydroxide
solution. The reaction mixture is then poured into a mixture of water and
sodium hydroxide solution, the precipitate is filtered off, dried and
recrystallised from benzene. The 2-methoxy-4,2'-dichloro-4'-nitrodiphenyl
ether obtained melts at 159°-161°C.
623 g of 2-methoxy-4,2'-dichloro-4'-nitrodiphenyl ether in 4000 ml of dioxan
are catalytically hydrogenated in the presence of 250 g of Raney nickel at
room temperature and under normal pressure. After the calculated amount of
hydrogen, the Raney nickel is filtered off and the 2-methoxy-4,2'-dichloro-4'-
aminodiphenyl ether is precipitated by the addition of water, filtered off,
washed and dried, melting point 100°-102°C.
204 g of well milled 2-methoxy-4,2'-dichloro-4'-aminodiphenyl ether are
added to a mixture of 254 ml of concentrated hydrochloric acid and 1600 ml
of water, the addition being made at room temperature while stirring well. The
suspension formed is cooled to 0°-5°C and at this temperature 225 g of 33%
sodium nitrite solution is added under the level of the liquid. The mixture is
stirred for another 12 h at 0°-5°C. A solution of 86 g of sodium bisulphate
and 60 g of sodium hydroxide in 640 ml of water is added at 80°C to a
solution of 400 g of crystallised copper sulfate and 106 g of sodium chloride in
1280 ml of water. The cuprous chloride formed is allowed to settle, the water
is poured off and the precipitate is purified by decanting three times with
water.
The residue is dissolved in 640 ml of concentrated hydrochloric acid, the
solution is heated to 65°-70°C and the diazo suspension produced above is
added while stirring. After cooling, the aqueous phase is poured off, the resin_x0002_like organic phase is taken up in ether, the ether solution is extracted with
dilute sodium hydroxide solution, washed neutral, dried over sodium sulphate
and concentrated. The residue is distilled under water jet vacuum. The 2-
methoxy-4,2',4'-trichlorodiphenyl ether obtained boils at 210°-217°C.
243 g of aluminum chloride are added to the solution of 187.5 g of 2-
methoxy-4,2',4'-trichlorodiphenyl ether in 800 ml of benzene and the reaction
mixture is boiled for 30 min while stirring. After cooling, it is poured into ice
and hydrochloric acid, the benzene phase is separated and extracted with
water and sodium hydroxide solution. The mimosa alkaline aqueous phase is
separated, the last remains of benzene are removed by blowing in steam, it is
then filtered and acidified with hydrochloric acid. The precipitated 2-hydroxy-
4,2',4'-tri-chlorodiphenyl ether is filtered off, washed and dried. After
recrystallisation from petroleum ether it melts at 60°-61°C.Model E., Bindler J.; GB Patent No. 1,051,823; Dec. 21, 1966; Assigned: J.R.
Geigy AG, Basel
brand name
Stri-Dex Cleansing Bar (Sterling Health U.S.A.); Stri-Dex Face Wash (Sterling Health U.S.A.).
Therapeutic Function
Antiseptic
General Description
Chemical structure: diphenyl ether derivative
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
Irgasan is a broad spectrum antimicrobial agent. It is an inhibitor of the enoyl-ACP (acyl-carrier protein) reductase component of type II fatty acid synthase (FAS-II) in bacteria and Plasmodium. It also inhibits mammalian fatty acid synthase (FASN), and may have anticarcinogenic activity.
Mechanism of action
Triclosan is active against a broad range of oral grampositive and gram-negative bacteria.The primary target of its antibacterial activity is the bacterial cell membrane. High concentrations cause membrane leakage and ultimately lysis of the bacterial cell. Effects at lower concentration are more subtle. Triclosan has been shown to bind to cell membrane targets and inhibit enzymes associated with the phosphotransferase and proton motive force systems.
Pharmacology
Triclosan is retained in dental plaque for at least 8 hours, which in addition to its broad antibacterial property could make it suitable for use as an antiplaque agent in oral care preparations. However, the compound is rapidly released from oral tissues, resulting in relatively poor antiplaque properties as assessed in clinical studies of plaque formation.This observation is further corroborated by a poor correlation between minimal inhibitory concentration values generated in vitro and clinical plaque inhibitory properties of triclosan. Improvement of substantivity was accomplished by incorporation of triclosan in a polyvinyl methyl ether maleic acid copolymer (PVM/MA, Gantrez). With the combination of PVM/MA copolymer and triclosan, the substantivity of the triclosan was increased to 12 hours in the oral cavity.
Clinical Use
Triclosan plus copolymer is available in toothpaste. Commercially available dentifrice concentrations contain 0.3% triclosan and 2.0% PVM/MA copolymer.
Side Effects
Triclosan is a preservative used in health care and consumer products, including soaps, deodorants, mouthwashes, toothpastes, cosmetics, and topical medicaments. Ozkaya et al. described a case of suspected immune mediated Cou to triclosan. A 44-year-old female reported experiencing an immediate localized urticarial response after contact with numerous topical products. The use of a toothpaste had also resulted in swelling of her lips, tongue, and breathing difficulties. She also experienced lip swelling after kissing her husband who had used the same product and wheals involving her face after kissing friends on the cheek who had used certain topical products on their faces. The suspected products all contained triclosan 0.2%–0.5%. A severe localized urticarial reaction occurred with open testing to 2% triclosan within 15 minutes. No tests were performed to confirm an immunological mechanism; however, the authors suspected this to be the case because of a positive urticarial response to triclosan within 15 minutes, a history of angioedema to the triclosan-containing toothpaste, and because no immediate reactions were seen in five control subjects who were open tested to 2% triclosan.
Safety Profile
Poison by intravenous andintraperitoneal routes. Moderately toxic by ingestion.Mildly toxic by skin contact. Mutation data reported. Ahuman skin irritant. When heated to decomposition itemits toxic fumes of Clí.
Veterinary Drugs and Treatments
Found in several products, often with other active ingredients, triclosan’s antibacterial effects may be useful in treating superficial
pyodermas.
Triclosan is a bis-phenol disinfectant/antiseptic. It has a activity against a wide range of organisms, including both gram-positive and
gram-negative bacteria and acts via inhibiting bacterial fatty acid synthesis leading to disruption of cell membrane integrity. Triclosan
reportedly is not effective against Pseudomonas spp. and may be less effective against staphylococci than either chlorhexidine or ethyl
lactate.
in vitro
triclosan blocked and displaced [3h] oestradiol binding from oestrogen receptors (er) of mcf7 human breast cancer cells and from recombinant human erα /erβ at low concentrations. triclosan fully dampened the elicitation of the oestrogen-responsive ere-cat reporter gene in mcf7 cells and the activation of growth of mcf7 human breast cancer cells by 10-10 m 17β-oestradiol. additionally, triclosan, on its own, increased the proliferation of oestrogen-dependent mcf7 human breast cancer cells [1].
in vivo
balb/c mice were administrated subcutaneously with triclosan daily at 0.8 to 38 mg/kg for 6 weeks. 75% parasitemia was blocked by single subcutaneous injection of triclosan at a dose of 3.0 mg/kg within 24 hours. however, triclosan fully cleared the parasite from circulation with one injection at a dose of 38 mg/kg. no side effects of triclosan were monitored via checking the activities of the enzymes serum glutamate oxaloacetate transaminase and serum glutamate pyruvate transaminase [2].
Definition
Triclosan [5-chloro-2-(2,4-dichlorophenoxy)phenol] is an antibacterial and antifungal agent used since the early 1970s. It was first described by E. Model and J. Bindler in 1964. It is used in consumer products ranging from toothpaste to deodorants to toys. The safety and effectiveness of triclosan recently have come under scrutiny, and the US Environmental Protection Agency and Food and Drug Administration have initiated efforts to reexamine its health risks.
Health Hazard
The antibacterial compound Triclosan has been linked to numerous human health problems. Exposures come mainly by absorption through the skin or through the lining of the mouth. These exposures have resulted in contact dermatitis, or skin irritation, and an increase in allergic reactions, especially in children. Triclosan has also been detected in human milk samples and urine at high concentrations, which correlates with the use pattern of this compound. Recent studies have also found that Triclosan interferes with the body’s thyroid hormone metabolism and may be a potential endocrine disruptor. Children exposed to antibacterial compounds at an early age also have an increased chance of developing allergies, asthma, and eczema.
Metabolism
Triclosan is prone to phase II metabolism via sulfotransferase and glucuronosyltransferase enzymes (Wang et al., 2004). In humans the resulting conjugates are excreted primarily in urine (Sandborgh-Englund et al., 2006).
References
[1]. gee, r., charles, a., taylor, n., & darbre, p. oestrogenic and androgenic activity of triclosan in breast cancer cells. journal of applied toxicology. 2007; 28(1): 78-91.
[2]. hillyer, c. triclosan offers protection against blood stages of malaria by inhibiting enoyl-acp reductase of plasmodium falciparumm. surolia, a. surolia. nat med 7:167–173, 2001. transfusion medicine reviews. 2002; 16(2): 180-181.
Properties of Triclosan
| Melting point: | 56-60 °C(lit.) |
| Boiling point: | 290°C(lit.) |
| Density | 1.4214 (rough estimate) |
| vapor pressure | 0.001Pa at 25℃ |
| refractive index | 1.4521 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble12g/L at 20°C |
| form | Solid |
| pka | 7.9(at 25℃) |
| color | colorless or white |
| Water Solubility | Soluble in ethanol, methanol, diethyl ether and sodium hydroxide solution (1M). Slightly soluble in water. |
| Merck | 14,9657 |
| BRN | 2057142 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 3380-34-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Triclosan(3380-34-5) |
| EPA Substance Registry System | Triclosan (3380-34-5) |
Safety information for Triclosan
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
Computed Descriptors for Triclosan
| InChIKey | XEFQLINVKFYRCS-UHFFFAOYSA-N |
Triclosan manufacturer
Alvika Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Triclosan 98%View Details
Triclosan 98%View Details -
 TRICLOSAN 99%View Details
TRICLOSAN 99%View Details -
 5-Chloro-2-(2,4-dichlorophenoxy)phenol CAS 3380-34-5View Details
5-Chloro-2-(2,4-dichlorophenoxy)phenol CAS 3380-34-5View Details
3380-34-5 -
 Triclosan USP BPView Details
Triclosan USP BPView Details
3380-34-5 -
 Triclosan U.S.PView Details
Triclosan U.S.PView Details
3380-34-5 -
 99% Triclosan APIView Details
99% Triclosan APIView Details
3380-34-5 -
 Dev Impex Triclosan, Treatment: Surgical Cleaning, PrescriptionView Details
Dev Impex Triclosan, Treatment: Surgical Cleaning, PrescriptionView Details
3380-34-5 -
 antibacterial and antifungal Triclosan, Grade: Technical, Packaging Size: 25 KgView Details
antibacterial and antifungal Triclosan, Grade: Technical, Packaging Size: 25 KgView Details
3380-34-5
