Trichlorine nitride
- CAS NO.:10025-85-1
- Empirical Formula: Cl3N
- Molecular Weight: 120.37
- EINECS: 233-045-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
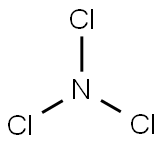
What is Trichlorine nitride?
Description
Trichloramine (NCl3), also known as nitrogen chloride or trichloride, is a toxic, explosive, nasty-smelling oily liquid with a boiling point of 71 oC. It is unstable in air and rapidly decomposes in water.
The earliest reported synthesis of NCl3?(Pierre Louis Dulong, 1813) was the reaction of molecular chlorine or sodium hypochlorite with ammonium salts. It can also be prepared from ammonia and chlorine and by the electrolysis of aqueous ammonium chloride.
NCl3’s primary commercial use was bleaching flour, but this use was banned in the United States in the late 1940s. It had been discovered in 1947 that eating bread made from NCl3-contaminated flour caused severe neurological disorders in humans and animals.
NCl3?is most famous (or infamous) for being the product of the reaction of chlorine in swimming pools with urea in urine or sweat. It is much more responsible for pools’ “chlorine smell” than chlorine itself.
In an?article?on disinfection byproducts in swimming pools related to the? Rio de Janeiro Olympic Games, Celia Henry Arnaud discusses NCl3?in connection with professional swimmers’ habit of intentionally peeing in pools. NCl3?can not only cause respiratory problems in swimmers, but it also can corrode metals in and around pools.
Mr. Phelps: Your mission, should you choose to accept it, is to discontinue this practice and urge other Olympians to do the same.
Chemical properties
Yellow oil or rhombic crystals.Insoluble in cold water; decomposes in hot water; soluble in chloroform, phosphorus trichloride, and carbon disulfide.
Physical properties
Yellow, oily, heavy liquid; pungent odor; density 1.653 g/mL; freezes to rhombohedral crystalline solid below -40°C; evaporates in air rapidly; vaporpressure 150 torr at 20°C; explodes when heated at 93°C; highly unstable, decomposes explosively in light; insoluble in water, decomposes slowly in cold water after several hours; decomposes in hot water; soluble in benzene, chloroform, carbon tetrachloride, carbon disulfide and phosphorus trichloride.
The Uses of Trichlorine nitride
Bleaching of flour; wastage control of citrus fruit.
Preparation
Nitrogen trichloride is prepared by passing chlorine gas into slightly acid solution of ammonium chloride. The product is continuously extracted with carbon tetrachloride:
NH4Cl + 3Cl2 → NCl3 + 4HCl
Hypochlorous acid, HOCl, also may be used instead of chlorine in such preparation.
Nitrogen trichloride can be prepared by the action of anhydrous chlorine with anhydrous ammonia:
3Cl2 + NH3 → NCl3 + 3HCl
Nitrogen trichloride is made commercially by electrolyzing an acidified solution of ammonium chloride.
Definition
ChEBI: Nitrogen trichloride is a nitrogen halide.
Hazard
Explodes when heated to approximately 200F (93C) or when exposed to direct sunlight. Toxic by ingestion and inhalation, strong irritant.
Safety Profile
Moderately toxic by inhalation. An irritant to the eyes, skin, mucous membranes, and a systemic central nervous system irritant. An explosive sensitive to impact, light, and ultrasound. The solid explodes on melting. The liquid explodes above 60°C. Concentrated solutions are also explosive. Explosive decomposition is initiated by contact with: concentrated ammonia, arsenic, dinitrogen tetraoxide, hydrogen sulfide, hydrogen trisulfide, nitrogen oxide, organic matter, ozone, phosphine, phosphorus, potassium cyanide, potassium hydroxide solutions, selenium, hydrogen chloride, hydrogen fluoride, hydrogen bromide, hydrogen iodide. mxtures with chlorine + hydrogen are potentially explosive. Upon decomposition it emits toxic fumes of Cl- and NOx. See also CHLORIDES.
Properties of Trichlorine nitride
| Melting point: | -27°C |
| Boiling point: | 71°C (estimate) |
| Density | 1.653 |
| solubility | insoluble in H2O; soluble in CS2, benzene, ctc 2 |
| pka | -22.67±0.70(Predicted) |
| form | yellow oily liquid |
| color | yellow oily liquid; explodes, explosive |
| Water Solubility | insoluble H2O, decomposes in H2O after 24h; soluble CS2, phosphorus trichloride, benzene, CCl4, CHCl3 [MER06] |
| CAS DataBase Reference | 10025-85-1 |
| EPA Substance Registry System | Nitrogen trichloride (10025-85-1) |
Safety information for Trichlorine nitride
Computed Descriptors for Trichlorine nitride
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
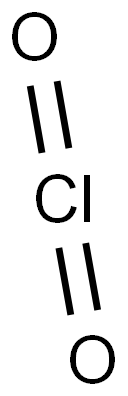
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
