Sodium bicarbonate
Synonym(s):Sodium bicarbonate;Sodium hydrogen carbonate;Sodium bicarbonate solution;aqueous sodium bicarbonate;soda bicarbonate
- CAS NO.:144-55-8
- Empirical Formula: CHNaO3
- Molecular Weight: 84.01
- MDL number: MFCD00003528
- EINECS: 205-633-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
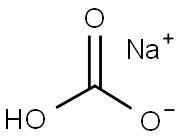
What is Sodium bicarbonate?
Description
Sodium bicarbonate, which is the compound commonly called baking soda, exists as a white,
odorless, crystalline solid. It occurs naturally as the mineral nahcolite, which derives its name
from its chemical formula by replacing the “3” in NaHCO3 with the ending “lite.” The world’s
main source of nahcolite is the Piceance Creek Basin in western Colorado, which is part of
the larger Green River formation. Sodium bicarbonate is extracted using solution mining by
pumping hot water through injection wells to dissolve the nahcolite from the Eocene beds
where it occurs 1,500 to 2,000 feet below the surface. The dissolved sodium bicarbonate is
pumped to the surface where it is treated to recover NaHCO3 from solution. Sodium bicarbonate
can also be produced from the trona deposits, which is a source of sodium carbonates
(see Sodium Carbonate).
Description
Sodium bicarbonate (NaHCO3) is commonly referred to as baking soda by people outside of chemistry. It is amphoteric, meaning it can neutralize both acids and bases. The most common use of NaHCO3 is as a saturated aqueous solution for performing a basic wash during an aqueous workup.
Chemical properties
Sodium bicarbonate occurs as an odorless, white, crystalline powder with a saline, slightly alkaline taste. The crystal structure is monoclinic prisms. Grades with different particle sizes, from a fine powder to free-flowing uniform granules, are commercially available.
Chemical properties
Sodium bicarbonate, NaHC03, also known as sodium acid carbonate and baking soda, is a white water-soluble crystalline solid.It has an alkaline taste, loses carbon dioxide at 270°C (518 °F).and is used in food preparation. Sodium bicarbonate also finds use as a medicine,a butter preservative, in ceramics,and to prevent timber mold.
Physical properties
White crystalline powder or granules; monoclinic crystals; density 2.20 g/cm3; decomposes around 50°C, begins to lose carbon dioxide; converts to sodium carbonate at 100°C; soluble in water, 10g/100 mL at 20°C; slowly decomposes to CO2 and Na2CO3 in aqueous solution at ambient temperature; decomposes to Na2CO3 in boiling water; aqueous solution slightly alkaline; pH of 0.1M solution at 25°C is about 8.3; insoluble in alcohol; decomposes in acids.
The Uses of Sodium bicarbonate
sodium bicarbonate (baking soda) is an inorganic salt used as a buffering agent and a pH adjuster, it also serves as a neutralizer. It is used in skin-smoothing powders.
The Uses of Sodium bicarbonate
manufacture of many sodium salts; source of CO2; ingredient of baking powder, effervescent salts and beverages; in fire extinguishers, cleaning Compounds.
The Uses of Sodium bicarbonate
Sodium Bicarbonate is a leavening agent with a ph of approxi- mately 8.5 in a 1% solution at 25°c. it functions with food grade phosphates (acidic leavening compounds) to release carbon dioxide which expands during the baking process to provide the baked good with increased volume and tender eating qualities. it is also used in dry-mix beverages to obtain carbonation, which results when water is added to the mix containing the sodium bicarbonate and an acid. it is a component of baking powder. it is also termed baking soda, bicarbonate of soda, sodium acid carbonate, and sodium hydrogen carbonate.
The Uses of Sodium bicarbonate
Sodium bicarbonate, used in the formof baking soda and baking powder, is the most common leavening agent. When baking soda,which is an alkaline substance, is added to a mix, it reacts with an acid ingredient to producecarbon dioxide. The reaction can be represented as: NaHCO3(s) + H+ → Na+(aq) + H2O(l) +CO2(g), where H+ is supplied by the acid. Baking powders contain baking soda as a primaryingredient along with acid and other ingredients. Depending on the formulation, bakingpowders can produce carbon dioxide quickly as a single action powder or in stages, as with adouble-action powder. Baking soda is also used as a source of carbon dioxide for carbonatedbeverages and as a buffer.In addition to baking, baking soda has numerous household uses. It is used as a generalcleanser, a deodorizer, an antacid, a fire suppressant, and in personal products such as toothpaste.Sodium bicarbonate is a weak base in aqueous solution, with a pH of about 8. Thebicarbonate ion (HCO3-) has amphoteric properties, which means it can act as either an acidor a base. This gives baking soda a buff ering capacity and the ability to neutralize both acidsand bases. Food odors resulting from acidic or basic compounds can be neutralized with bakingsoda into odor-free salts. Because sodium bicarbonate is a weak base, it has a greater abilityto neutralize acid odors.
The second largest use of sodium bicarbonate, accounting for approximately 25% of totalproduction, is as an agricultural feed supplement. In cattle it helps maintain rumen pH andaids fiber digestibility; for poultry it helps maintain electrolyte balance by providing sodiumin the diet, helps fowl tolerate heat, and improves eggshell quality.
Sodium bicarbonate is used in the chemical industry as a buff ering agent, a blowingagent, a catalyst, and a chemical feedstock. Sodium bicarbonate is used in the leather tanningindustry for pretreating and cleaning hides and to control pH during the tanning process.Heating sodium bicarbonate produces sodium carbonate, which is used for soap and glassmaking.Sodium bicarbonate is incorporated into pharmaceuticals to serve as an antacid, abuff ering agent, and in formulations as a source of carbon dioxide in eff ervescent tablets. Drychemical type BC fire extinguishers contain sodium bicarbonate (or potassium bicarbonate).Other uses of bicarbonate include pulp and paper processing, water treatment, and oil welldrilling.
Indications
Sodium bicarbonate is used for the treatment of metabolic acidosis which may occur in severe renal disease, uncontrolled diabetes, circulatory insufficiency due to shock or severe dehydration, extracorporeal circulation of blood, cardiac arrest and severe primary lactic acidosis. Also is indicated in severe diarrhea which is often accompanied by a significant loss of bicarbonate.
Further indicated in the treatment of certain drug intoxications, including barbiturates (where dissociation of the barbiturateprotein complex is desired), in poisoning by salicylates or methyl alcohol and in hemolytic reactions requiring alkalinization of the urine to diminish nephrotoxicity of blood pigments.
Production Methods
Sodium bicarbonate is manufactured either by passing carbon dioxide into a cold saturated solution of sodium carbonate, or by the ammonia–soda (Solvay) process, in which first ammonia and then carbon dioxide is passed into a sodium chloride solution to precipitate sodium bicarbonate while the more soluble ammonium chloride remains in solution.
Definition
Baking soda: A white solid formed either by passing an excess of carbon dioxide through sodium carbonate or hydroxide solution, or by precipitation when cold concentrated solutions of sodium chloride and ammonium hydrogencarbonate are mixed. Sodium hydrogencarbonate decomposes on heating to give sodium carbonate, carbon dioxide, and water. With dilute acids, it yields carbon dioxide. It is used as a constituent of baking powder, in effervescent beverages, and in fire extinguishers. Its aqueous solutions are alkaline as a result of salt hydrolysis. Sodium hydrogencarbonate forms monoclinic crystals.
Production Methods
Most sodium bicarbonate in the United States is made synthetically by the reaction of sodium carbonate solution (Na2CO3) with carbon dioxide: Na2CO3(aq) + H2O(l) + CO2(g) → 2NaHCO3(aq). It can also be produced using the Solvay process, which uses ammonia, carbon dioxide, and salt to produce sodium bicarbonate according to the following series of reactions: 2NH3(g) + CO2(g) + H2O(l) → (NH4)2CO3(aq)
(NH4)2CO3(aq) + CO2(g) + H2O(l) → 2NH3HCO3(aq)
NH4HCO3(aq) + NaCl(aq) → NaHCO3(s) + NH4Cl(aq).
brand name
Neut (Abbott); Soda Mint (Lilly).
General Description
Odorless white crystalline powder or lumps. Slightly alkaline (bitter) taste. pH (of freshly prepared 0.1 molar aqueous solution): 8.3 at 77°F. pH (of saturated solution): 8-9. Non-toxic.
Air & Water Reactions
Stable in dry air, but slowly decomposes in moist air. Moderately water soluble. Decomposes slowly in water (accelerated by agitation) .
Reactivity Profile
Sodium bicarbonate reacts exothermically with acids to generate non-toxic carbon dioxide gas. Decomposes when heated. Incompatible with acids, acidic salts (dopamine hydrochloride, pentazocine lactate, many alkaloidal salts) aspirin and bismuth salicylate.
Fire Hazard
Literature sources indicate that Sodium bicarbonate is noncombustible.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Sodium bicarbonate is generally used in pharmaceutical formulations
as a source of carbon dioxide in effervescent tablets and
granules. It is also widely used to produce or maintain an alkaline
pH in a preparation.
In effervescent tablets and granules, sodium bicarbonate is
usually formulated with citric and/or tartaric acid; combinations
of citric and tartaric acid are often preferred in formulations as citric
acid alone produces a sticky mixture that is difficult to granulate,
while if tartaric acid is used alone, granules lose firmness. When the
tablets or granules come into contact with water, a chemical
reaction occurs, carbon dioxide is evolved, and the product disintegrates. Melt granulation in a fluidized bed dryer has been
suggested as a one-step method for the manufacture of effervescent
granules composed of anhydrous citric acid and sodium bicarbonate,
for subsequent compression into tablets.
Tablets may also be prepared with sodium bicarbonate alone
since the acid of gastric fluid is sufficient to cause effervescence and
disintegration. Sodium bicarbonate is also used in tablet formulations
to buffer drug molecules that are weak acids, thereby
increasing the rate of tablet dissolution and reducing gastric
irritation.
The effects of tablet binders, such as polyethylene glycols,
microcrystalline cellulose, silicified microcrystalline cellulose, pregelatinized
starch, and povidone, on the physical and mechanical
properties of sodium bicarbonate tablets have also been investigated.(
8,9)
Additionally, sodium bicarbonate is used in solutions as a
buffering agent for erythromycin, lidocaine, local anesthetic
solutions, and total parenteral nutrition (TPN) solutions. In
some parenteral formulations, e.g. niacin, sodium bicarbonate is
used to produce a sodium salt of the active ingredient that has
enhanced solubility. Sodium bicarbonate has also been used as a
freeze-drying stabilizer and in toothpastes.
Recently, sodium bicarbonate has been used as a gas-forming
agent in alginate raft systems and in floating, controlledrelease
oral dosage forms for a range of drugs. Tablet
formulations containing sodium bicarbonate have been shown to
increase the absorption of paracetamol, and improve the
stability of levothyroxine. Sodium bicarbonate has also been
included in formulations of vaginal bioadhesive tablets and in
carbon dioxide releasing suppositories.
Therapeutically, sodium bicarbonate may be used as an antacid,
and as a source of the bicarbonate anion in the treatment of
metabolic acidosis. Sodium bicarbonate may also be used as a
component of oral rehydration salts and as a source of bicarbonate
in dialysis fluids; it has also been suggested as a means of preventing
radiocontrast-induced nephrotoxicity.
Sodium bicarbonate is used in food products as an alkali or as a
leavening agent, e.g. baking soda.
Pharmaceutical Applications
Sodium bicarbonate is usually administered orally in order to regulate the serum pH. Imbalances of the plasma pH can be due to problems occurring in the kidneys such as renal tubular acidosis. Within the kidneys, blood is filtered before it passes through the tubular part of the nephrons where re-absorption or secretion of important salts and others takes place. In renal tubular acidosis, the kidneys either fail to filter or secrete acid ions (H+) from the plasma (secretion takes place in the distal tubule), or to recover bicarbonate ions (HCO3-) from the filtrate (passive re-absorption takes place in the proximal tubule, active re-absorption at the distal tubule), which is necessary to balance the pH. In the view of this mode of action, the pharmaceutically active component of sodium bicarbonate is the bicarbonate anion, but the cation Na+ is responsible for solubility and compatibility.
Biological Activity
Commonly used laboratory reagent
Biochem/physiol Actions
Sodium bicarbonate can be used to treat salicylate poisoning.
Pharmacokinetics
Intravenous sodium bicarbonate therapy increases plasma bicarbonate, buffers excess hydrogen ion concentration, raises blood pH and reverses the clinical manifestations of acidosis.
Clinical Use
Metabolic acidosis
Alkalinisation of urine
Renoprotection against contrast media
Safety Profile
Low toxicity by ingestion. An experimental teratogen. A nuisance dust. Human systemic effects: changes in potassium levels, increased urine volume, metabolic acidosis, nausea or vomiting, respiratory changes, sodium level changes. Mutation data reported.
Safety
Sodium bicarbonate is used in a number of pharmaceutical
formulations including injections and ophthalmic, otic, topical,
and oral preparations.
Sodium bicarbonate is metabolized to the sodium cation, which
is eliminated from the body by renal excretion, and the bicarbonate
anion, which becomes part of the body’s bicarbonate store. Any
carbon dioxide formed is eliminated via the lungs. Administration
of excessive amounts of sodium bicarbonate may thus disturb the
body’s electrolyte balance, leading to metabolic alkalosis or possibly
sodium overload with potentially serious consequences. The
amount of sodium present in antacids and effervescent formulations
has been sufficient to exacerbate chronic heart failure, especially in
elderly patients.
Orally ingested sodium bicarbonate neutralizes gastric acid with
the evolution of carbon dioxide and may cause stomach cramps and
flatulence.
When used as an excipient, sodium bicarbonate is generally
regarded as an essentially nontoxic and nonirritant material.
LD50 (mouse, oral): 3.36 g/kg
LD50 (rat, oral): 4.22 g/kg
Veterinary Drugs and Treatments
Sodium bicarbonate is indicated to treat metabolic acidosis and alkalinize the urine. It is also used as adjunctive therapy in treating hypercalcemic or hyperkalemia crises.
Drug interactions
Potentially hazardous interactions with other drugs
Increases lithium excretion.
Metabolism
Oral bicarbonate, such as sodium bicarbonate, neutralises gastric acid with the production of carbon dioxide. Bicarbonate not involved in that reaction is absorbed and in the absence of a deficit of bicarbonate in the plasma, bicarbonate ions are excreted in the urine, which is rendered alkaline, and there is an accompanying diuresis.
storage
When heated to about 50℃, sodium bicarbonate begins to
dissociate into carbon dioxide, sodium carbonate, and water; on
heating to 250–300℃, for a short time, sodium bicarbonate is
completely converted into anhydrous sodium carbonate. However,
the process is both time- and temperature-dependent, with
conversion 90% complete within 75 minutes at 93°C. The reaction proceeds via surface-controlled kinetics; when sodium bicarbonate
crystals are heated for a short period of time, very fine needleshaped
crystals of anhydrous sodium carbonate are formed on the
sodium bicarbonate surface.
The effects of relative humidity and temperature on the moisture
sorption and stability of sodium bicarbonate powder have been
investigated. Sodium bicarbonate powder is stable below 76%
relative humidity at 25℃ and below 48% relative humidity at
40℃. At 54% relative humidity, the degree of pyrolytic
decarboxylation of sodium bicarbonate should not exceed 4.5%
in order to avoid detrimental effects on stability.
At ambient temperatures, aqueous solutions slowly decompose
with partial conversion into the carbonate; the decomposition is
accelerated by agitation or heat. Aqueous solutions begin to break
up into carbon dioxide and sodium carbonate at about 20°C, and
completely on boiling.
Aqueous solutions of sodium bicarbonate may be sterilized by
filtration or autoclaving. To minimize decomposition of sodium
bicarbonate by decarboxylation on autoclaving, carbon dioxide is
passed through the solution in its final container, which is then
hermetically sealed and autoclaved. The sealed container should not
be opened for at least 2 hours after it has returned to ambient
temperature, to allow time for the complete reformation of the
bicarbonate from the carbonate produced during the heating process.
Aqueous solutions of sodium bicarbonate stored in glass
containers may develop deposits of small glass particles. Sediments
of calcium carbonate with traces of magnesium or other metal
carbonates have been found in injections sterilized by autoclaving;
these are due to impurities in the bicarbonate or to extraction of
calcium and magnesium ions from the glass container. Sedimentation
may be retarded by the inclusion of 0.01–0.02% disodium
edetate.
Sodium bicarbonate is stable in dry air but slowly decomposes in
moist air and should therefore be stored in a well-closed container in
a cool, dry place.
Safety
Sodium bicarbonate is likely safe when used appropriately, short-term. Over-the-counter antacid products containing sodium bicarbonate are considered safe and effective by the US FDA. Taking sodium bicarbonate in very high doses is possibly unsafe. It is also possibly unsafe to take sodium bicarbonate that it is not fully dissolved into a solution. Stomach rupture and serious changes in electrolyte levels have occurred.
Purification Methods
Crystallise it from hot water (6mL/g). The solid should not be heated above 40o due to the formation of carbonate.
Incompatibilities
Sodium bicarbonate reacts with acids, acidic salts, and many
alkaloidal salts, with the evolution of carbon dioxide. Sodium
bicarbonate can also intensify the darkening of salicylates.
In powder mixtures, atmospheric moisture or water of crystallization
from another ingredient is sufficient for sodium bicarbonate
to react with compounds such as boric acid or alum. In liquid
mixtures containing bismuth subnitrate, sodium bicarbonate reacts
with the acid formed by hydrolysis of the bismuth salt.
In solution, sodium bicarbonate has been reported to be
incompatible with many drug substances such as ciprofloxacin, amiodarone, nicardipine, and levofloxacin.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (injections; ophthalmic preparations; oral capsules, solutions, and tablets). Included in parenteral (intravenous infusions and injections) and nonparenteral medicines (chewing gums; ear drops; eye lotions; oral capsules, chewable tablets, effervescent powders, effervescent tablets, granules, soluble tablets, orodispersible tablets, and tablets; suppositories and suspensions) licensed in the UK.
Properties of Sodium bicarbonate
| Melting point: | >300 °C(lit.) |
| Boiling point: | 851°C |
| Density | 2.16 g/mL at 25 °C (lit.) |
| refractive index | 1.500 |
| storage temp. | 2-8°C |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | solution (7.5%) |
| appearance | White crystalline solid |
| color | White |
| Specific Gravity | 2.159 |
| PH | 8.27(1 mM solution);8.22(10 mM solution);8.02(100 mM solution); |
| pka | (1) 6.37, (2) 10.25 (carbonic (at 25℃) |
| PH Range | 7.8 - 8.2 |
| Odor | Odorless |
| Water Solubility | 9 g/100 mL (20 ºC) |
| Decomposition | 50 °C |
| Merck | 14,8583 |
| BRN | 4153970 |
| Stability: | Stable. |
| CAS DataBase Reference | 144-55-8(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium bicarbonate (144-55-8) |
Safety information for Sodium bicarbonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Sodium bicarbonate
Sodium bicarbonate manufacturer
Bondbay Pharmaceuticals Pvt Ltd
Benzer Multitech India Pvt Ltd
Sudeep Pharma Pvt Ltd
Ennor Muds and Chemicals
ARRAKIS INDUSTRIES LLP
New Products
1-Amino-1-cyclohexanecarboxylic acid 2-Hydroxyacetamide Ethyl 5-cyclopropyl-1H-pyrazole-3-carboxylate 2,4,6-Trichloroaniline ELECTROLYTIC IRON POWDER 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate N,N'-diallyl-1,3-diaminopropanedihydrochloride 3-Iodo-6-nitroindazole (4-(4-Fluorophenoxy)phenyl)methanamine Tert-butyl N-(pent-4-yn-2-yl) carbamate 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine 2-chloro-4-methyl-5-nitro pyridine 2,5-dibromopyridine terephthalonitrile 5-Bromo-4-chloro-2(1h)-pyridinone, 5-Fluoro-2-Oxindole N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA]Related products of tetrahydrofuran
You may like
-
 Sodium Bi Carbonate 99%View Details
Sodium Bi Carbonate 99%View Details -
 Sodium Bi Carbonate 99%View Details
Sodium Bi Carbonate 99%View Details -
 Sodium hydrogen carbonate, 98% 99%View Details
Sodium hydrogen carbonate, 98% 99%View Details -
 Sodium bicarbonate 99%View Details
Sodium bicarbonate 99%View Details -
 Sodium bicarbonate 98%View Details
Sodium bicarbonate 98%View Details -
 SODIUM BICARBONATE 98%View Details
SODIUM BICARBONATE 98%View Details -
 Sodium hydrogen carbonate CAS 144-55-8View Details
Sodium hydrogen carbonate CAS 144-55-8View Details
144-55-8 -
 sodium bicarbonate CASView Details
sodium bicarbonate CASView Details