Sodium dichloroisocyanurate
Synonym(s):3,5-Dichloro-2-hydroxy-4,6-s-triazinedione sodium salt;Dichloro-s-triazinetrione sodium salt;NaDCC
- CAS NO.:2893-78-9
- Empirical Formula: C3Cl2N3NaO3
- Molecular Weight: 219.95
- MDL number: MFCD00006036
- EINECS: 220-767-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
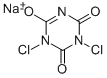
What is Sodium dichloroisocyanurate?
Chemical properties
Dichloroisocyanuric acid, sodium salt, is a white crystalline powder. Chlorine odor. Thermally unstable. It is produced as a result of reaction of cyanuric acid with chlorine. Sodium dichloroisocyanurate is a well established disinfectant used for many purposes including wound cleansing, hospital use, sterilizing babies bottles, disinfection of water for human consumption and disinfection of swimming pools.
Characteristics
Sodium dichloroisocyanurate (NaDCC) is a broad-spectrum disinfectant agent:
More stable than bleach (sodium hypochlorite) - consistent strength produced at point of use for cleaning and disinfecting hard surfaces;
Almost neutral in pH - less corrosive on surfaces than liquid bleach;
Biodegradable – safe for the environment.
The Uses of Sodium dichloroisocyanurate
Sodium Dichloroisocyanurate (SDIC) widely applied for the sterilization of swimming pool and drinking water, or fighting against infectious diseases, or act as disinfectant in raising silkworm, livestock, poultry and fish. Other applications of SDIC are found in wool shrinkage, textile bleaching, and industrial circulating water cleaning. SDIC is normally supplied in powder and granular, tablets are also available on request. Stabilised chlorine granular (dichlor) are used very widely to chlorinate swimming pool water.
The Uses of Sodium dichloroisocyanurate
Sodium dichloroisocyanurate can be used as a reagent for N-monochlorination and dehydrochlorination of amino esters. It is also a reagent for chlorination to detect ammonium via formation of colored zebra-bands in a detecting tube.
What are the applications of Application
Sodium dichloroisocyanurate is a versatile reagent
Preparation
Sodium dichloroisocyanurate is produced by chlorination of disodium cyanurate [Na2H(NCO)3] using chlorine (CI2) and neutralization with sodium hydroxide (NaOH).
Disodium cyanurate is obtained by action of sodium hydroxide on isocyanuric acid.
General Description
White solid with an odor of bleach-like odor. Mixes with water.
Air & Water Reactions
Water soluble. May vigorously react with water releasing chlorine gas. Material containing less than 39% available chlorine will undergo reactions as described herein, but may take longer to initiate, and the resulting reaction may not be as vigorous [AAR 1992].
Reactivity Profile
Contact with ammonium compounds or hydrated salts can cause a very vigorous reaction. Prolonged exposure to heat /fire may result in the vigorous decomposition of the material with the rupture of its containers, Sodium dichloroisocyanurate will accelerate the burning of combustible materials [AAR 1991]. Chlorine plus alcohols would yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M 1991].
Hazard
Strong oxidizing material, fire risk near organic materials. Toxic by ingestion.
Health Hazard
Dust causes sneezing and coughing, moderate irritation of the eyes, and itchiness and redness of the skin. Ingestion causes burns of mouth and stomach.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic to humans and animals by ingestion. An experimental teratogen. Experimental reproductive effects. A severe skin and eye irritant. Human systemic effects by ingestion: ulceration or bleeding from stomach. The other main toxic effects were gastrointestinal irritation, salivation, lachrymation, dyspnea, weakness, emaciation, lethargy, diarrhea, coma, and (following very high dosage) death after 1-8 days, with autopsy showing irritation of stomach and gastrointestinal tract, liver dysfunction, and lung congestion. The concentrated material may be a little more toxic, due to greater gastrointestinal irritation. In the dry form, it is not appreciably irritating to dry skin. However, when moist, the concentrated material is irritating to skin, and also may cause severe eye irritation. A powerful oxidizer. Incompatible with combustible materials, ammonium salts, nitrogenous materials. Used to chlorinate swimming pools and in cleaning, bleaching, disinfecting, sanitizing. When heated to decomposition it emits very toxic fumes of Cl-, NOx, and Na2O.
Potential Exposure
Dichloroisocyanuric acid salts, are used in cleaning; making dry bleaches, detergents, sanitizers, and disinfectants; in swimming pool and sewage treatment.
Shipping
UN2465 Dichloroisocyanuric acid, dry or Dichloroisocyanuric acid salts, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Incompatibilities
A powerful oxidizer. Dust may form explosive mixture with air. Violent reaction with reducing agents; organic matter; easily chlorinated or oxidized materials. Isocyanates are highly flammable and reactive with many compounds, even with themselves. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Reaction with moist air, water or alcohols may form amines and insoluble polyureas and react exothermically, releasing toxic, corrosive or flammable gases, including carbon dioxide; and, at the same time, may generate a violent release of heat increasing the concentration of fumes in the air. Incompatible with amines, aldehydes, alkali metals, ammonia, carboxylic acids, caprolactum, alkaline materials, glycols, ketones, mercaptans, hydrides, organotin catalysts, phenols, strong acids, strong bases, strong reducing agents such as hydrides, urethanes, ureas. Elevated temperatures or contact with acids, bases, tertiary amines, and acyl-chlorides may cause explosive polymerization. Contact with metals may evolve flammable hydrogen gas. Attacks some plastics, rubber and coatings May accumulate static electrical charges, and may cause ignition of its vapors. Incompatible with ammonium salts, amines forming nitrogen trichloride
Properties of Sodium dichloroisocyanurate
| Melting point: | 225°C |
| Density | 1 g/cm3 |
| vapor pressure | 0.006Pa at 20℃ |
| storage temp. | 0-6°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated, Sonicated), Water |
| form | powder to lump |
| color | White to Almost white |
| Water Solubility | 30g/100ml (25 ºC) |
| Sensitive | Moisture Sensitive |
| BRN | 4056155 |
| Stability: | Stable. Oxidizing agent - contact with combustible material may lead to fire. Incompatible with strong bases, strong oxidizing agents. Reacts readily with many nitrogen-containing compounds to form explosive nitrogen triiodide. Moisture-sensitive. |
| InChI | InChI=1S/C3HCl2N3O3.Na/c4-7-1(9)6-2(10)8(5)3(7)11;/h(H,6,9,10);/q;+1/p-1 |
| EPA Substance Registry System | Sodium dichloro-s-triazinetrione (2893-78-9) |
Safety information for Sodium dichloroisocyanurate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H302:Acute toxicity,oral H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium dichloroisocyanurate
| InChIKey | MSFGZHUJTJBYFA-UHFFFAOYSA-M |
| SMILES | C1(=O)N(Cl)C(=NC(=O)N1Cl)[O-].[Na+] |
Sodium dichloroisocyanurate manufacturer
Benzer Multitech India Private Limited
Acuro Organics Limited
Madan Minerals & Chemicals Industries
NRS Chemicals LLP
Antares Chem Private Limited
Zeel Product
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Sodium Dichloroisocyanurate CASView Details
Sodium Dichloroisocyanurate CASView Details -
 Dichloroisocyanuric acid sodium salt CAS 2893-78-9View Details
Dichloroisocyanuric acid sodium salt CAS 2893-78-9View Details
2893-78-9 -
 Chlorine Powder Sodium Dichloroisocyanurate, Packaging Size: 50, Sheet Thickness: IndustrialView Details
Chlorine Powder Sodium Dichloroisocyanurate, Packaging Size: 50, Sheet Thickness: IndustrialView Details
2893-78-9 -
 Technical Grade Sodium Dichloroisocyanurate Sdic, For Industrial, Packaging Size: 50kgView Details
Technical Grade Sodium Dichloroisocyanurate Sdic, For Industrial, Packaging Size: 50kgView Details
2893-78-9 -
 Powder Sodium Dichloroisocyanurate (SDIC), Packaging Type: Drum, Packaging Size: 40 kgView Details
Powder Sodium Dichloroisocyanurate (SDIC), Packaging Type: Drum, Packaging Size: 40 kgView Details
2893-78-9 -
 Sodium Dichloroisocyanurate Powder, Physical State: GranulesView Details
Sodium Dichloroisocyanurate Powder, Physical State: GranulesView Details
2893-78-9 -
 Sdic Sodium Dichloroisocyanurate, Physical State: GranulesView Details
Sdic Sodium Dichloroisocyanurate, Physical State: GranulesView Details
2893-78-9 -
 Sodium Dichloroisocyanurate PowderView Details
Sodium Dichloroisocyanurate PowderView Details
28-39-7
