Triamterene
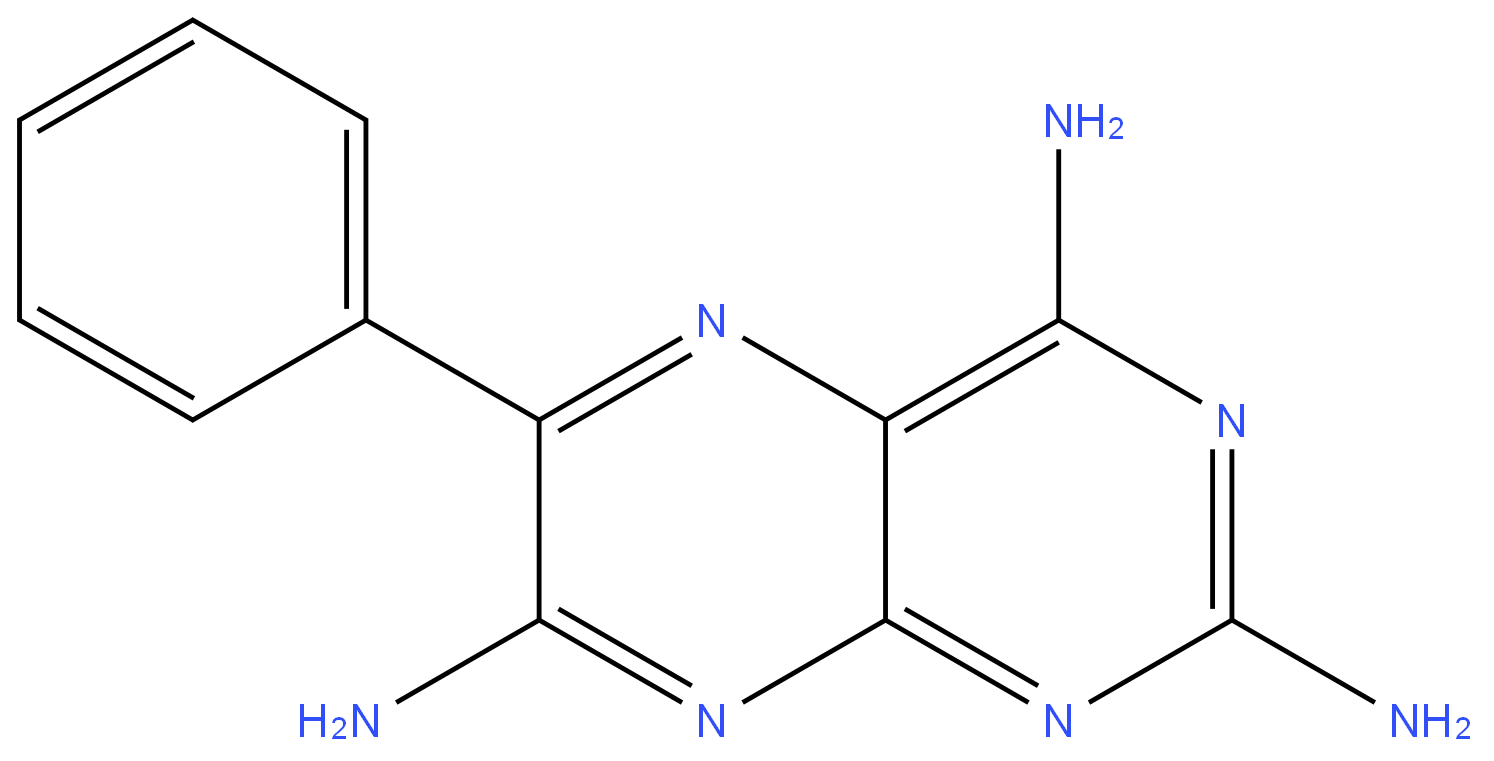
Synonym(s):2,4,7-Triamino-6-phenylpteridine;6-Phenyl-2,4,7-pteridinetriamine;Triamterene
- CAS NO.:396-01-0
- Empirical Formula: C12H11N7
- Molecular Weight: 253.26
- MDL number: MFCD00006708
- EINECS: 206-904-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
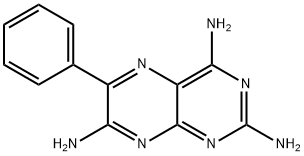
What is Triamterene?
Absorption
Triamterene is shown to be rapidly absorbed in the gastrointestinal tract Its onset of action achiveved within 2 to 4 hours after oral ingestion and its duration of action is 12-16 hours. It is reported that the diuretic effect of triamterene may not be observed for several days after administration. In a pharmacokinetic study, the oral bioavailability of triamterene was determined to be 52%. Following administration of a single oral dose to fasted healthy male volunteers, the mean AUC of triamterene was about 148.7 ng*hr/mL and the mean peak plasma concentrations (Cmax) were 46.4 ng/mL reached at 1.1 hour after administration. In a limited study, administration of triamterene in combination with hydrochlorothiazide resulted in an increased bioavailability of triamterene by about 67% and a delay of up to 2 hours in the absorption of the drug. It is advised that triamterene is administered after meals; in a limited study, combination use of triamterene and hydrochlorothiazide with the consumption of a high-fat meal resulted in an increase in the mean bioavailability and peak serum concentrations of triamterene and its active sulfate metabolite, as well as a delay of up to 2 hours in the absorption of the active constituents.
Toxicity
Acute oral LD50 of triamterene in rats is 400 mg/kg and 285-380 mg/kg in mice. There has been a case of reversible acute renal failure following ingestion of 50 combination pills containing 50 mg triamterene and 25 mg hydrochlorothiazide. Symptoms of overdose, such as nausea, vomiting, gastrointestinal disturbances, weakness, and hypotension, are related to electrolyte imbalances, such as hyperkalemia. As there is no specific antidote, emesis and gastric lavage should be use to induce immediate evacuation of the stomach and careful evaluation of the electrolyte pattern and fluid balance should be made. Dialysis may be somewhat effective in case of an overdosage.
In a carciongenicity study in male and female mice administered with triamterene at the highst dosage level, there was an increased incidence of hepatocellular neoplasia, primarily adenomas. However, this was not a dose-dependent phenomenon and there was no statistically significant difference from control incidence at any dose level. In bacterial assays, there was no demonstrated mutagenic potential of triamterene. In in vitro assay using Chinese hamster ovary (CHO) cells with or without metabolic activation, there were no chromosomal aberrations. Studies evaluating the effects of triamterene on reproductive system or fertility have not been conducted. It is advised that the use of triamterene is avoided during pregnancy. As triamterene has been detected in human breast milk, triamterene should be used when nursing is ceased.
Description
Triamterene is an inhibitor of the epithelial sodium channel (ENaC; IC50 = 4.5 μM for the rat channel). In vivo, triamterene (0.5-32 mg/animal) enhances sodium secretion and decreases potassium secretion in adrenalectomized rats. Formulations containing triamterene have been used in the treatment of edema. This product is also available as an analytical reference standard .
Chemical properties
Yellow Solid
Originator
Jatropur,Rohm,W. Germany,1962
The Uses of Triamterene
Triamterene is a potassium-sparing diuretic ie, it inhibits the urinary excretion of potassium
The Uses of Triamterene
Triamterene is a weak diuretic with potassium sparing properties; blocks Na+ reuptake in the kidneys.
The Uses of Triamterene
This drug is recommended in combination with other diuretics for treating edema caused by usual reasons such as circulatory insufficiency, cirrhosis of the liver, and nephrotic syndrome.
What are the applications of Application
Triamterene is an Na+ channel blocker
Background
Triamterene (2,4,7-triamino-6-phenylpteridine) is a potassium-sparing diuretic that is used in the management of hypertension. It works by promoting the excretion of sodium ions and water while decreasing the potassium excretion in the distal part of the nephron in the kidneys by working on the lumenal side. Since it acts on the distal nephron where only a small fraction of sodium ion reabsorption occurs, triamterene is reported to have limited diuretic efficacy. Due to its effects on increased serum potassium levels, triamterene is associated with a risk of producing hyperkalemia. Triamterene is a weak antagonist of folic acid, and a photosensitizing drug.
Triamterene was approved by the Food and Drug Administration in the U.S. in 1964. Currently, triamterene is used in the treatment of edema associated with various conditions as monotherapy and is approved for use with other diuretics to enhance diuretic and potassium-sparing effects. It is also found in a combination product with hydrochlorothiazide that is used for the management of hypertension or treatment of edema in patients who develop hypokalemia on hydrochlorothiazide alone.
Indications
Triamterene is indicated for the treatment of edema associated with congestive heart failure, cirrhosis of the liver, and the nephrotic syndrome; also in steroid-induced edema, idiopathic edema, and edema due to secondary hyperaldosteronism.
Triamterene in combination with hydrochlorothiazide is indicated for the managment of hypertension or treatment of edema in patients who develop hypokalemia following hydrochlorothiazide monotherapy, and in patients who require thiazide diuretic and in whom the development of hypokalemia cannot be risked. Triamterene allows the maintenance of potassium balance when given in combination with loop diuretics and thiazides.
Definition
ChEBI: Triamterene is pteridine substituted at positions 2, 4 and 7 with amino groups and at position 6 with a phenyl group. A sodium channel blocker, it is used as a diuretic in the treatment of hypertension and oedema. It has a role as a diuretic and a sodium channel blocker.
Manufacturing Process
To a solution of 9 grams of 5-nitroso-2,4,6-triaminopyrimidine in 500 mi of
refluxing dimethylformamide is added 9 grams of phenylacetonitrile and the
refluxing is stopped. The 3 grams of anhydrous sodium methoxide is added
and the mixture is refluxed for 15 minutes. The mixture is chilled and the
solid is filtered and washed several times with warm water until the washings
are neutral. Drying gives yellow crystals which are recrystallized with a Darco
treatment from formamide-water heating the solution no hotter than 125°C.
This product is then suspended in filtered deionized water and warmed for 15
minutes. This yields the 2,4,7-triamino-6-phenylpteridine as yellow crystals
with a MP of 314° to 317°C.
Therapeutic Function
Diuretic
Biological Functions
Triamterene (Dyrenium) results in changes in urinary electrolyte patterns that are qualitatively similar to those produced by spironolactone. The mechanism by which this agents bring about the alterations in electrolyte loss, however, is quite different. Triamterene produces this effects whether or not aldosterone or any other mineralocorticoid is present. The action of this drug is clearly unrelated to endogenous mineralocorticoid activity, and this drug is effective in adrenalectomized patients.
General Description
Odorless yellow powder or crystalline solid. Melting point 316°C. Almost tasteless at first and with a slightly bitter aftertaste. Acidified solutions give a blue fluorescence. Used as a diuretic drug.
Air & Water Reactions
Sensitive to light; slowly oxidized upon exposure to air. Insoluble in water.
Reactivity Profile
2,4,7-Triamino-6-phenylpteridine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Fire Hazard
Flash point data for 2,4,7-Triamino-6-phenylpteridine are not available; however, 2,4,7-Triamino-6-phenylpteridine is probably combustible.
Mechanism of action
Triamterene is a pyrazine derivative that inhibits reabsorption of sodium ions without
increasing excretion of potassium ions. It exhibits the same approximate effect as spironolactone;
however, it does not competitively bind with aldosterone receptors. Its action does
not have an effect on secretion of aldosterone or its antagonists, which are a result of direct
action on renal tubules.
This potassium sparing diuretic causes a moderate increase in excretion of sodium and
bicarbonate ions in urine, and it raises excretion of potassium and ammonia ions. It has little
effect on urine volume.
Pharmacokinetics
Triamterene, a relatively weak, potassium-sparing diuretic and antihypertensive, is used in the management of hypertension and edema. It primarily works on the distal nephron in the kidneys; it acts from the late distal tubule to the collecting duct to inhibit Na+ reabsorption and decreasing K+ excretion. As triamterene tends to conserve potassium more strongly than promoting Na+ excretion, it can cause an increase in serum potassium, which may result in hyperkalemia potentially associated with cardiac irregularities. In healthy volunteers administered with oral triamterene, there was an increase in the renal clearnace of sodium and magnesium, and a decrease in the clearance of uric acid and creatinine due to its effect of reducing glomerular filtration renal plasma flow. Triamterene does not affect calcium excretion. In clinical trials, the use of triamterene in combination with hydrochlorothiazide resulted an enhanced blood pressure-lowering effects of hydrochlorothiazide.
Clinical Use
Triamterene can be used in the treatment of congestive
heart failure, cirrhosis, and the edema caused by
secondary hyperaldosteronism. It is frequently used in
combination with other diuretics except spironolactone.
Amiloride, but not triamterene, possesses antihypertensive
effects that can add to those of the thiazides.
These K+-sparing diuretics have low efficacy when
used alone, since only a small amount of total Na reabsorption
occurs at more distal sites of the nephron.
These compounds are used primarily in combination
with other diuretics, such as the thiazides and loop diuretics,
to prevent or correct hypokalemia. The availability
of fixed-dose mixtures of thiazides with nonsteroidal
K+-sparing compounds has proved a rational
form of drug therapy. Both triamterene and amiloride
are available alone or in combination with hydrochlorothiazide.
Side Effects
Because the actions of triamterene and amiloride are independent of plasma aldosterone levels, their prolonged administration is likely to result in hyperkalemia. Both amiloride and triamterene are contraindicated in patients with hyperkalemia.Triamterene should not be given to patients with impaired renal function. Potassium intake must be reduced, especially in outpatients.A folic acid deficiency has been reported to occur occasionally following the use of triamterene.
Synthesis
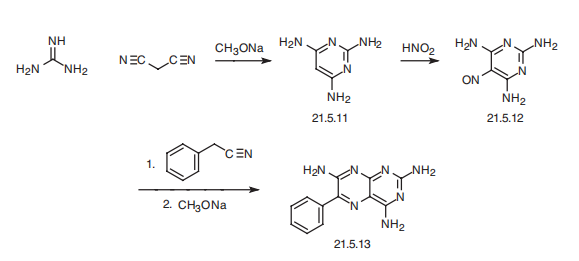
Triamterene, 2,4,7-triamino-6-phenylpteridine (21.5.13), is synthesized in by the following scheme. Reacting guanidine with malonodinitrile gives 2,4,6-triaminopyrimidine (21.5.11). This undergoes nitrosation by reacting it with nitric acid, which results in the formation of 5-nitroso-2,4,6-triaminopyrimidine (21.5.12), which upon condensation with benzyl cyanide in the presence of sodium methoxide cyclizes into triamterene (21.5.13).
Veterinary Drugs and Treatments
Triamterene is a potassium-sparing diuretic that potentially could be used as an alternative to spironolactone for the adjunctive treatment of congestive heart failure in dogs, however, there is little experience associated with its use in dogs or cats.
Drug interactions
Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
enhanced hypotensive effect (risk of severe
hyperkalaemia).
Analgesics: increased risk of nephrotoxicity with
NSAIDs; increased risk of hyperkalaemia, especially
with indometacin; antagonism of hypotensive effect.
Antibacterials: avoid concomitant use with
lymecycline.
Antidepressants: enhanced hypotensive effect with
MAOIs; increased risk of postural hypotension with
tricyclics.
Antipsychotics: enhanced hypotensive effect with
phenothiazines.
Antihypertensives: enhanced hypotensive effect;
increased risk of first dose hypotensive effect of postsynaptic alpha-blockers, e.g. prazosin.
Ciclosporin: increased risk of hyperkalaemia.
Cytotoxics: increased risk of nephrotoxicity and
ototoxicity with platinum compounds.
Lithium: reduced excretion of lithium (risk of
lithium toxicity).
Potassium salts: increased risk of hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia.
Metabolism
Triamterene undergoes phase I metabolism involving hydroxylation, via CYP1A2 activity, to form 4'-hydroxytriamterene. 4'-Hydroxytriamterene is further transformed in phase II metabolism mediated by cytosolic sulfotransferases to form the major metabolite, 4′-hydroxytriamterene sulfate, which retains a diuretic activity. Both the plasma and urine levels of this metabolite greatly exceed triamterene levels while the renal clearance of the sulfate conjugate was les than that of triamterene; this low renal clearance of the sulfate conjugate as compared with triamterene may be explained by the low unbound fraction of the metabolite in plasma.
Metabolism
Triamterene is extensively metabolised apparently via the cytochrome P450 isoenzyme CYP1A2. It is mainly excreted in the urine in the form of metabolites with some unchanged triamterene; variable amounts are also excreted in the bile.
Properties of Triamterene
| Melting point: | 316°C |
| Boiling point: | 386.46°C (rough estimate) |
| Density | 1.3215 (rough estimate) |
| refractive index | 1.8260 (estimate) |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | formic acid: soluble200 mg + 4 mL warm Formic Acid, clear, yellow-green |
| pka | 6.2(at 25℃) |
| form | Solid |
| form | neat |
| color | Pale Yellow to Yellow |
| Water Solubility | <0.1 G/100 ML AT 20 ºC |
| Merck | 14,9599 |
| BRN | 266723 |
| CAS DataBase Reference | 396-01-0(CAS DataBase Reference) |
| IARC | 2B (Vol. 108) 2016 |
| NIST Chemistry Reference | Triamterene(396-01-0) |
| EPA Substance Registry System | Triamterene (396-01-0) |
Safety information for Triamterene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Triamterene
| InChIKey | FNYLWPVRPXGIIP-UHFFFAOYSA-N |
Triamterene manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Triamterene 98% (HPLC) CAS 396-01-0View Details
Triamterene 98% (HPLC) CAS 396-01-0View Details
396-01-0 -
 Triamterene 98% (HPLC) CAS 396-01-0View Details
Triamterene 98% (HPLC) CAS 396-01-0View Details
396-01-0 -
 Triamterene CAS 396-01-0View Details
Triamterene CAS 396-01-0View Details
396-01-0 -
 Triamterene CAS 396-01-0View Details
Triamterene CAS 396-01-0View Details
396-01-0 -
 Triamterene CAS 396-01-0View Details
Triamterene CAS 396-01-0View Details
396-01-0 -
 396-01-0 Triamterene 98%View Details
396-01-0 Triamterene 98%View Details
396-01-0 -
 396-01-0 98%View Details
396-01-0 98%View Details
396-01-0 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1