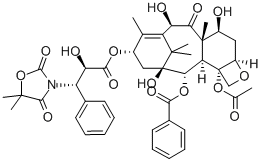
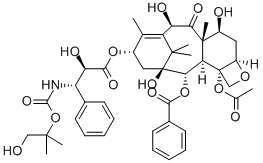
Docetaxel
Synonym(s):Docetaxel;Docetaxel trihydrate
- CAS NO.:114977-28-5
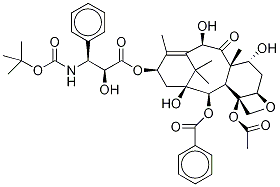
- Empirical Formula: C43H53NO14
- Molecular Weight: 807.88
- MDL number: MFCD00871399
- EINECS: 601-339-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
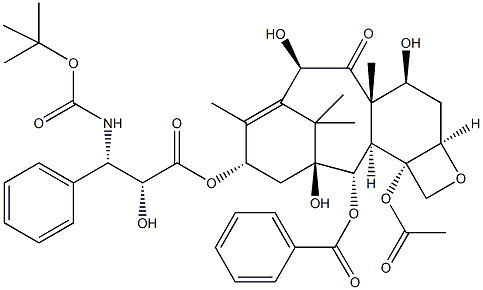
What is Docetaxel?
Absorption
The pharmacokinetic profile of docetaxel is consistent with a three-compartment model. The initial rapid decline represents the distribution to the peripheral compartments, and the late (terminal) phase is partly due to a relatively slow efflux of docetaxel from the peripheral compartment. The area under the curve (AUC) was dose proportional at doses between 70 mg/m2 and 115 mg/m2 with infusion times of 1 to 2 hours. In a group of patients with solid tumors given 100 mg/m2 of docetaxel intravenously, the Cmax and AUC were 2.41 μg/mL and 5.93 μg?h/mL, respectively.
Toxicity
There is no known antidote for an overdose of docetaxel injection. In case of overdose, patients should be closely monitored in specialized units. Some of the anticipated complications of overdosage include: bone marrow suppression, peripheral neurotoxicity, and mucositis. After an overdose is discovered, patients should receive granulocyte colony-stimulating factor (G-CSF) as soon as possible. Other appropriate symptomatic measures should be taken as needed.
In two reports of overdose, one patient received 150 mg/m2, and the other received 200 mg/m2 as 1-hour infusions. Both patients experienced severe neutropenia, mild asthenia, cutaneous reactions, and mild paresthesia, and recovered without incident. In rats, the oral LD50 of docetaxel is >2000 mg/kg. The intravenous LD50 in mice is 138 mg/kg.
Description
Docetaxel, a semi-synthetic member of the taxoid family, was introduced to the market in 1995, initially in South Africa and subsequently in various other markets, primarily for treating ovarian, breast, and non-small cell lung cancers. Similar to the naturally occurring antitumor agent paclitaxel, which was the first taxoid to be marketed, docetaxel facilitates tubulin assembly into stable microtubules and impedes their depolymerization, acting as a mitotic spindle poison and inducing a mitotic block in proliferating cells. This mechanism distinguishes taxoids from other classes of anticancer agents. Docetaxel has demonstrated twice the potency of paclitaxel in numerous in vitro assays and exhibits heightened cytotoxicity. Ongoing clinical trials are exploring its efficacy in treating other types of tumors, including pancreatic, gastric, head and neck cancers, and soft tissue sarcomas.
Chemical properties
Off-white Cryst
Originator
Rhone-Poulenc Rorer (France)
The Uses of Docetaxel
Docetaxel is a semisynthetic analog of taxol that inhibits microtubule disassembly (IC50 = 0.2 μM) and inhibits cell replication (IC50 = 0.13 μM). It has proven more effective than taxol in preventing the proliferation of cancer cells. Docetaxel has applications in breast cancer and hormone-refractory prostate cancer. This product is intended for research applications.
The Uses of Docetaxel
antineoplastic;binds to microtubules
The Uses of Docetaxel
Docetaxel used to treat variety of cancers (Lung, Breast, Prostate). It is a second-generation cytotoxic antimicrotubule agent and is mostly used in the pharmaceutical industry.
Indications
Docetaxel is indicated as a single agent for the treatment of locally advanced or metastatic breast cancer after chemotherapy failure; and with doxorubicin and cyclophosphamide as adjuvant treatment of operable node-positive BC. It is also indicated as a single agent for locally advanced or metastatic non-small cell lung cancer (NSCLC) after platinum therapy failure; and with cisplatin for unresectable, locally advanced or metastatic untreated NSCLC. For the treatment of metastatic castration-resistant prostate cancer, docetaxel is indicated with prednisone. Docetaxel is also indicated with cisplatin and fluorouracil for untreated, advanced gastric adenocarcinoma, including the gastroesophageal junction, and with cisplatin and fluorouracil for induction treatment of locally advanced squamous cell carcinoma of the head and neck (SCCHN).
Background
Docetaxel, a clinically established anti-mitotic chemotherapy medication, is utilized for treating various cancers, including breast, ovarian, and non-small cell lung cancer. As a complex diterpenoid molecule and a semisynthetic analogue of paclitaxel, docetaxel binds reversibly to microtubulin with high affinity in a 1:1 stoichiometric ratio. This interaction inhibits cell division and promotes cell death. Unlike paclitaxel, docetaxel demonstrates twice the potency as an inhibitor of microtubule depolymerization. Notably, it binds to microtubules but does not interact with dimeric tubulin. Despite its efficacy, the use of docetaxel may lead to undesired outcomes, including hepatic impairment, hematologic effects, enterocolitis, neutropenic colitis, hypersensitivity reactions, fluid retention, second primary malignancies, embryo-fetal toxicity, and tumor lysis syndrome. Approved by the FDA in 1996, docetaxel is available in solution for injection for intravenous or parenteral administration.
Definition
ChEBI: A tetracyclic diterpenoid that is paclitaxel with the N-benzyloxycarbonyl group replaced by N-tert-butoxycarbonyl, and the acetoxy group at position 10 replaced by a hydroxy group.
Manufacturing Process
Taxol, a material occurring in nature, and extracted from Taxus brevifolia (i.e.
the Pacific yew tree). It consists of the A, B and C variants. Taxol is not water
soluble, thereby complicating its delivery in vivo for therapeutic purposes.
A sample of Taxol (14.7 g, 17 mmol) was dissolved in pyridine (150 mL) and
chlorotriethylsilane (23.03 g, 147 mmol) was added. The reaction was stirred
at 25°C under N2. After 20 hours the reaction appeared complete by TLC
analysis (7% MeOH/CH2Cl2). The mixture was concentrated to remove the
pyridine. The residue was dissolved in CH2Cl2 and washed with water, 10%
CuSO4, NaHCO3 and brine successively. The organic layer was dried over
MgSO4, and concentrated to yield 20.89 g of the crude 2,7'-bis(triethylsilyl)
Taxol. A portion of crude 2',7-bis(triethylsilyl) Taxol (14.50 g, 13.4 mmol) was
dissolved in dry THF (150 mL). Zirconocene chloride hydride (7.75 g, 30.2
mmol) was added. The reaction was stirred at 25°C under N2. After 20 hours
the reaction appeared complete by TLC analysis. The mixture was poured intocold hexanes, and the resulting precipitated Zr complexes were filtered off.
The solution was concentrated to yield 17 g of the crude 2,7'-bis(triethylsilyl)
Taxol imine. A portion of crude 2',7-bis(triethylsilyl) Taxol imine (8.36 g) was
dissolved in 1% HCl/EtOH (180 mL) and the reaction was stirred at 25°C for
20 hours. The reaction appeared complete by TLC analysis. The mixture was
poured into 800 mL of water and washed with hexane (180 mL times 3). The
aqueous layer was neutralized with NaHCO3 to pH=7.0. The product was
extracted with CH2Cl2. The organic layer was removed and concentrated to a
solid. Silica gel chromatography (5% MeOH/CH2Cl2) yielded Taxol primary
amine (2.41 g, 52% overall yield based on 5 g of Taxol used). Melting point
160°C-162°C.
A sample of Taxol primary amine (100 mg, 0.13 mmol) was dissolved in
CH2Cl2 (10 mL) and HCl (15 mM in Et2O; 10 ml, 150 mmol) was added. The
reaction was stirred at 25°C for 2 minutes. The mixture was concentrated to
remove the solvents. The residue was redissolved in CH2Cl2 and precipitated
in hexane. Filtration yielded 85 mg of Taxol PA (PA-primary amine) HCl
(83%). Melting point 65°C. A sample of Taxol PA HCl (50 mg, 0.064 mmol)
was dissolved in 0.5 ml of water. It was neutralized to pH 7.0 by addition of
saturated NaHCO3, followed by extraction with CH2Cl2. The organic layer was
concentrated and chromatographed (3% MeOH/CH2Cl2was used as mobile
phase) to yield 30 mg of Taxol primary amine (63% yield). The 1H NMR and
LRMS data agree well with a standard sample of Taxol primary amine.
Trimethylsilyl- and trichlorethoxycarbonyl-protecting group can be used. A
mixture of impure Taxol A, B, C can be converted Taxol primary amine, which
then can be converted to Taxol A or docetaxel.
brand name
Taxotere (Sanofi Aventis).
Therapeutic Function
Antitumor
Hazard
A poison. Human systemic effects.
Pharmacokinetics
Docetaxel, classified as a taxoid antineoplastic agent, functions by facilitating the assembly of microtubules from tubulin dimers and stabilizing them, thus preventing depolymerization. This stabilization disrupts the normal dynamic reorganization of the microtubule network crucial for vital interphase and mitotic cellular functions. Moreover, docetaxel induces the formation of abnormal microtubule arrays or "bundles" throughout the cell cycle and multiple asters of microtubules during mitosis. However, the use of docetaxel may pose risks, including treatment-related deaths in patients with breast cancer and non-small cell lung cancer, hepatic impairment, hematologic effects, enterocolitis, and neutropenic colitis. Additional potential adverse effects include hypersensitivity reactions, fluid retention, the development of second primary malignancies, cutaneous reactions, neurologic reactions, eye disorders, asthenia, embryo-fetal toxicity, and tumor lysis syndrome.
Clinical Use
Antineoplastic agent:
Treatment of breast cancer, prostate cancer and
non-small cell lung cancer unresponsive to alternative
therapies, also gastric adenocarcinoma, squamous cell
carcinoma of head and neck
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly increased by
clarithromycin - avoid or reduce docetaxel dose
Antifungals: concentration possibly increased by
itraconazole and voriconazole - avoid or reduce
docetaxel dose.
Antipsychotics: avoid with clozapine - increased risk
of agranulocytosis.
Antivirals: concentration possibly increased by
indinavir, ritonavir and saquinavir avoid or reduce
docetaxel dose.
Ciclosporin: possibly inhibits metabolism of
ciclosporin; bioavailability of docetaxel increased by
ciclosporin.
Metabolism
Docetaxel undergoes hepatic metabolism. In vitro drug interaction studies revealed that docetaxel is metabolized by the CYP3A4 isoenzyme. CYP3A5 also plays a role in the metabolism of this drug. In humans, docetaxel is metabolized by CYP3A4/5 into four metabolites: M1, M2, M3 and M4. Docetaxel undergoes hydroxylation of the synthetic isobutoxy side chain, forming metabolite M2. The oxidation of M2 forms an unstable aldehyde that is immediately cyclised into the stereoisomers M1 and M3. M4 is then formed by the oxidation of M1/M3.
Metabolism
A study of [14C]-docetaxel has been conducted in three cancer patients. Docetaxel was eliminated in both the urine and faeces following cytochrome P450 3A4-mediated oxidative metabolism of the tert-butyl ester group, within seven days, the urinary and faecal excretion accounted for about 6% and 75% of the administered radioactivity, respectively. About 80% of the radioactivity recovered in faeces is excreted during the first 48 hours as one major inactive metabolite and 3 minor inactive metabolites and very low amounts of unchanged medicinal product.
Storage
Store at +4°C
References
1) Fabbri et al. (2008), Mitotic catastrophe and apoptosis induced by docetaxel in hormone-refractory prostate cancer cells; J. Cell Physiol, 217 494 2) Dosso and Berthold (2008), Docetaxel in the management of prostate cancer: current standard of care and future directions; Expert Opin. Pharmacother, 9 1969 3) Homma et al. (2008), RPN2 gene confers docetaxel resistance in breast cancer; Nat. Med., 14 939 4) Kars et al. (2008), Reversal of Multidrug Resistance by Synthetic and Natural Compounds in Drug-Resistant MCF-7 Cell Lines; Chemotherapy, 54 194 5) Wallin et al. (2012), GDC-0941, A Novel Class I Selective PI3K Inhibitor, Enhances the Efficacy of Docetaxel in Human Breast Cancer Models by Increasing Cell Death In vitro and In vivo; Clin Cancer Res.,?18 3901 6) Heinemann et al. (2011), Synergistic effects of oncolytic reovirus and docetaxel chemotherapy in prostate cancer; BMC Cancer, 11 221
Properties of Docetaxel
| Melting point: | 186-192 °C (dec.) |
| Boiling point: | 900.5±65.0 °C(Predicted) |
| alpha | -36 º (c=0.74,EtOH) |
| Density | 1.38 |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Practically insoluble in water, freely soluble in anhydrous ethanol, soluble in methylene chloride. |
| form | neat |
| pka | 11.20±0.46(Predicted) |
| form | Solid |
| color | White |
| Water Solubility | Soluble in dimethyl sulfoxide and ethanol. Insoluble in water. |
| Merck | 14,3397 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| CAS DataBase Reference | 114977-28-5(CAS DataBase Reference) |
Safety information for Docetaxel
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H341:Germ cell mutagenicity H362:Reproductive toxicity, effects on or via lactation H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Docetaxel
| InChIKey | BEDLLNJKXXVTSX-LWWLJZAUSA-N |
Docetaxel manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Docetaxel 99%View Details
Docetaxel 99%View Details -
 114977-28-5 Docetaxel 98%View Details
114977-28-5 Docetaxel 98%View Details
114977-28-5 -
 114977-28-5 98%View Details
114977-28-5 98%View Details
114977-28-5 -
 Docetaxel 99%View Details
Docetaxel 99%View Details -
 Docetaxel 95% CAS 114977-28-5View Details
Docetaxel 95% CAS 114977-28-5View Details
114977-28-5 -
 Docetaxel, 97%+ CAS 114977-28-5View Details
Docetaxel, 97%+ CAS 114977-28-5View Details
114977-28-5 -
 Docetaxel CAS 114977-28-5View Details
Docetaxel CAS 114977-28-5View Details
114977-28-5 -
 Docetaxel (120mg) Fresenius Kabi India Pvt Ltd Daxotel 120 Mg Injection, 3ml VialView Details
Docetaxel (120mg) Fresenius Kabi India Pvt Ltd Daxotel 120 Mg Injection, 3ml VialView Details
114977-28-5
