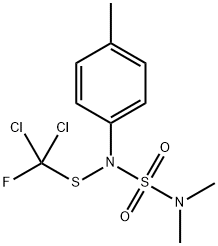
TOLYLFLUANID
- CAS NO.:731-27-1
- Empirical Formula: C10H13Cl2FN2O2S2
- Molecular Weight: 347.26
- MDL number: MFCD00055477
- EINECS: 211-986-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
What is TOLYLFLUANID?
The Uses of TOLYLFLUANID
Tolylfluanid is used to control a wide range of fungal diseases on apples, grapes, strawberries and other fruit and storage diseases on many crops.
The Uses of TOLYLFLUANID
Tolylfluanid may be used as a reference standard for the determination of the analyte in nutraceutical formulations using dispersive liquid-liquid microextraction(DLLME) coupled with gas chromatography-mass spectrometry method.
Definition
ChEBI: A member of the class of sulfamides that is dichlofluanid in which the hydrogen at the para position of the phenyl group is replaced by a methyl group. A fungicide first marketed in 1971 and used in the cultivation of fruit and vegetables, as ell as in wood preservatives, it is no longer approved for use in the European Union.
Metabolic pathway
Tolylfluanid contains an unstable dichlorofluoromethylthio (sulfenyl) moiety that has been shown to undergo rapid hydrolytic and metabolic degradation to dimethylaminosulfotoluidide (2). By analogy with captan, presumably the dichlorofluoromethylthio moiety can be transferred to the sulfur atoms of cellular thiols such as cysteine and glutathione. Thus, in the presence of thiols tolylfluanid is probably cleaved at the N-S bond to form thiophosgene or its monofluoro analogue (3) and other gaseous products such as hydrogen sulfide, hydrogen chloride and carbonyl sulfide. Thiophosgene or its monofluoro analogue is rapidly hydrolysed by water. The dichlorofluoromethylthio group and thiophosgene may be intermediates in the formation of addition products, for example addition to cysteine affords thiazolidine-2-thione-4-carboxylica cid (4). A thiazolidine derivative of glutathione may also be formed. The main metabolic reactions in all media were cleavage of the N-S bond to give dimethylaminosulfotoluidide (2) followed by hydroxylation of the phenyl ring and oxidation of the methyl substituent on the phenyl ring. Glucoside conjugates were detected in plants and the cysteine adduct, thiazolidine-2- thione-4-carboxylic acid (4), was formed in rats.
Degradation
Tolylfluanid is hydrolysed rapidly in alkaline conditions. The hydrolytic DT50 is about 12 days, 29 hours and <10 minutes at pH 4,7 and 9, respectively, at 22 °C. In the environment, hydrolysis is faster than photolysis (PM; PSD, 1995). Tolylfluanid does not absorb light of wavelength >295 nm and so photodegradation is unlikely to occur in the absence of photosensitisers.
Properties of TOLYLFLUANID
| Melting point: | 96°C |
| Boiling point: | 93℃ |
| Density | 1.5132 (rough estimate) |
| vapor pressure | 2 x 10-5 Pa (20 °C) |
| refractive index | 1.6000 (estimate) |
| storage temp. | -20°C |
| solubility | soluble in DMSO, Methanol |
| pka | -5.06±0.50(Predicted) |
| Colour Index | 45430 |
| form | neat |
| Water Solubility | 0.9 mg l-1 (20 °C) |
| BRN | 2949607 |
| EPA Substance Registry System | Tolylfluanid (731-27-1) |
Safety information for TOLYLFLUANID
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H372:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for TOLYLFLUANID
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran
You may like
-
 Tolylfluanid CAS 731-27-1View Details
Tolylfluanid CAS 731-27-1View Details
731-27-1 -
 Tolylfluanid CAS 731-27-1View Details
Tolylfluanid CAS 731-27-1View Details
731-27-1 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 7439-89-6 Electrolytic Iron Flakes 99.9% MaxView Details
7439-89-6 Electrolytic Iron Flakes 99.9% MaxView Details
7439-89-6 -
 7439-89-6 98.0% MinView Details
7439-89-6 98.0% MinView Details
7439-89-6 -
 Reduced Iron Powder 99.8% MaxView Details
Reduced Iron Powder 99.8% MaxView Details
7439-89-6 -
 Electrolytic Iron Powder 7439-89-6 99.8% MaxView Details
Electrolytic Iron Powder 7439-89-6 99.8% MaxView Details
7439-89-6