CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Colorless odorless solid; [HSDB] White to pale yellow crystalline solid; [MSDSonline] |
|---|---|
| Color/Form | Colorless crystals |
| Odor | Odorless |
| Boiling Point | Decomposes on distillation |
| Melting Point | 93 °C |
| Solubility | Solubility in toluene: 0.9 g/mL (20 °C), 1.06 g/mL (25 °C), 1.2 g/mL (30 °C); hexane: 0.03 g/mL (20 °C), 0.05 g/mL (25 °C), 0.05 g/mL (30 °C); miscible in all proportions with acetone, ethanol, ethyl acetate, methylene chloride. |
| Density | 1.52 g/cu cm |
| Vapor Pressure | 0.0000015 [mmHg] |
| LogP | log Kow = 3.90 at 20 °C |
| Henry's Law Constant | Henry's Law constant = 7.6X10-7 atm-cu m/mol at 25 °C /Estimated/ |
| Stability/Shelf Life | Hydrolysis DT50 (22 °C) 12 days (pH 4), 29 hr (pH 7), <10 min (pH 9). Under environmental conditions, hydrolysis occurs much more rapidly than photolysis. |
| Decomposition | Decomposes on distillation. |
| Ionization Efficiency | Positive |
| Collision Cross Section | 175.32 Ų [M+Na]+ |
| Kovats Retention Index | 2016 2073 2013.8 2037.4 2031.2 2016.9 |
| Other Experimental Properties | Hydroxyl radical reaction rate constant = 1.8X10-11 cu cm/molecule-sec at 25 °C /Estimated/ |
| Chemical Classes | Pesticides -> Fungicides |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H372:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 347.3 g/mol |
|---|---|
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 345.9779536 g/mol |
| Monoisotopic Mass | 345.9779536 g/mol |
| Topological Polar Surface Area | 74.3 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 393 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
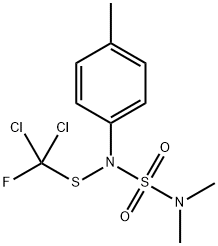
Tolylfluanid is a member of the class of sulfamides that is dichlofluanid in which the hydrogen at the para position of the phenyl group is replaced by a methyl group. A fungicide first marketed in 1971 and used in the cultivation of fruit and vegetables, as well as in wood preservatives, it is no longer approved for use in the European Union. It has a role as a genotoxin and an antifungal agrochemical. It is an organofluorine compound, an organochlorine compound, a member of sulfamides and a phenylsulfamide fungicide. It is functionally related to a sulfamide.