Sulfamide
- CAS NO.:7803-58-9
- Empirical Formula: H4N2O2S
- Molecular Weight: 96.11
- MDL number: MFCD00011606
- EINECS: 232-262-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-18 19:10:02
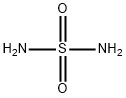
What is Sulfamide?
Chemical properties
White Solid
The Uses of Sulfamide
Sulfamide and its derivatives can also be used to prepare fused thiadiazine systems, Reaction with 2-aminoacetophenones affords IH-2,1,3-benzothia- diazine 2,2-dioxides 77 (65JOC3960). The corresponding 3,4-dihydro deriv- atives are readily available from 2-aminobenzylamines with either sulfuryl chloride (70M1443) or sulfamide (66USP3278532; 71MI055). These kinds of compounds can also be obtained from N-aryl-N'-alkylsulfamides and s-trioxane in a reaction involving cyclization by intramolecular sulfamido- methylation (79JOC2032) (Scheme 30).
The Uses of Sulfamide
A carbonic anhydrase inhibitor; used for treatment of cancer.
The Uses of Sulfamide
Similar to urea in many of its reactions, but is more acidic, and can act as a dibasic acid.
Definition
ChEBI: The simplest of the sulfamic acids consisting of a single sulfur atom covalently bound by single bonds to two amino groups and by double bonds to two oxygen atoms.
Preparation
Sulfamide is produced by the reaction of sulfuryl chloride with ammonia.
Purification Methods
Crystallise sulfamide from absolute EtOH. It decomposes at 250o. [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 482-483 1963.]
Properties of Sulfamide
| Melting point: | 90-92 °C(lit.) |
| Density | 1.611 g/mL at 25 °C(lit.) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | water: soluble50mg/mL, clear, colorless to faintly yellow |
| form | Crystalline Powder, Free From Visible Impurities |
| pka | 10.87±0.60(Predicted) |
| color | White to practically white , free from visible impurities |
| PH | 5.67 at 21.7℃ and 10g/L |
| Water Solubility | soluble |
| Sensitive | Moisture Sensitive |
| Merck | 14,8922 |
| CAS DataBase Reference | 7803-58-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Sulfamide(7803-58-9) |
| EPA Substance Registry System | Sulfamide (7803-58-9) |
Safety information for Sulfamide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sulfamide
| InChIKey | NVBFHJWHLNUMCV-UHFFFAOYSA-N |
Sulfamide manufacturer
Deea Pharmaseuticals LLP
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

![[3-[[[2-(DiaMinoMethyleneaMino)-4-thiazolyl]Methyl]thio]propionyl]sulfaMide Hydrochloride
(FaMotidine IMpurity)](https://img.chemicalbook.in/CAS/GIF/76824-17-4.gif)
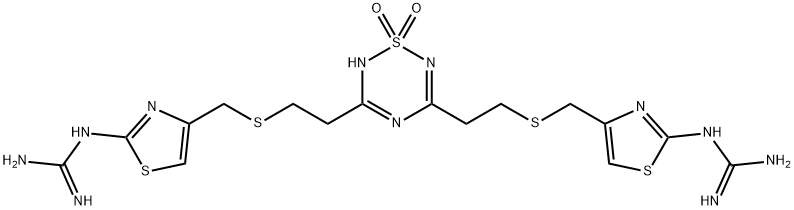
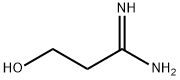
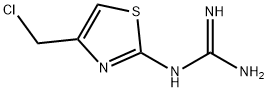
![3-[2-(Diaminomethyleneamino)-1,3-thiazol-4-ylmethylsulphinyl]-N-sulphamoyl
propanamide](https://img.chemicalbook.in/CAS2/GIF/1020719-36-1.gif)
You may like
-
 Sulfamide CAS 7803-58-9View Details
Sulfamide CAS 7803-58-9View Details
7803-58-9 -
 Sulfamide 98% CAS 7803-58-9View Details
Sulfamide 98% CAS 7803-58-9View Details
7803-58-9 -
 Sulfamide CAS 7803-58-9View Details
Sulfamide CAS 7803-58-9View Details
7803-58-9 -
 Sulfamide CAS 7803-58-9View Details
Sulfamide CAS 7803-58-9View Details
7803-58-9 -
 S0890 Sulfamide APIView Details
S0890 Sulfamide APIView Details
7803-58-9 -
 CAS 7803-58-9 H4N2O2S Sulfamide, Deea Pharmaseuticals LLP, Packaging Size: 25 KgView Details
CAS 7803-58-9 H4N2O2S Sulfamide, Deea Pharmaseuticals LLP, Packaging Size: 25 KgView Details
7803-58-9 -
 Powder Sulfamide, 7803-58-9View Details
Powder Sulfamide, 7803-58-9View Details
7803-58-9 -
 Sulfamide Cas No. 7803-58-9View Details
Sulfamide Cas No. 7803-58-9View Details
7803-58-9
