Tivozanib
- CAS NO.:475108-18-0
- Empirical Formula: C22H19ClN4O5
- Molecular Weight: 454.86
- MDL number: MFCD15146788
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
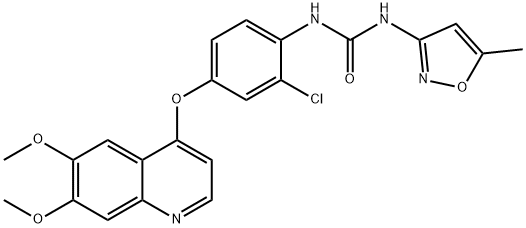
What is Tivozanib?
Absorption
The median Tmax of tivozanib is 10 hours, however, can range from 3 to 24 hours. A pharmacokinetic study in 8 healthy subjects revealed a Cmax and AUC for radiolabeled tivozanib of 12.1 ± 5.67 ng/mL and 1084 ± 417.0 ng·h/mL, respectively. Steady-state tivozanib concentrations are achieved at concentrations 6-7 times higher the normal dose.
Toxicity
LD50 information for tivozanib is not readily available in the literature, however the European Medicines Agency (EMA) assessment report indicates the oral maximum tolerated dose (MTD) in mice was 524 mg/kg; doses ≥ 750 mg/kg led to severe and lethal effects. In rats, the oral MTD was to 276 mg/kg; at doses ≥ 369 mg/kg, severe effects and death occurred.
In tivozanib monotherapy studies, two patients received overdoses of tivozanib. One volunteer with a prior history of hypertension experienced aggravated uncontrolled hypertension that resulted in death after taking 3 doses of 1340 microgram tivozanib in one day (total of 4020 micrograms). A second patient patient ingested two doses of 1340 microgram tivozanib in one day (total of 2680 micrograms), and experienced no adverse effects. Carefully control blood pressure before starting tivozanib; regularly monitor blood pressure during treatment. In the case of a confirmed or suspected overdose, discontinue tivozanib, monitor the patient closely, and provide supportive treatment as necessary. No antidote exists for an overdose with tivozanib.
Characteristics
Primary targets: VEGFR/multikinase
Class: receptor tyrosine kinase
Treatment: RCC
Elimination half-life = 111 h
Protein binding = >99%
The Uses of Tivozanib
Tivozanib also known as AV-951 is an orally bioavailable potent VEGFR-1, 2 and 3, c-Kit and PDGFR inhibitor with IC50 of 0.21, 0.16, 0.24, 1.63 and 1.72 nM, respectively.
The Uses of Tivozanib
Tivozanib is known as an oral VEGF receptor tyrosine kinase inhibitor, exhibiting antitumor effects towards renal cell carcinoma.. Tivozanib suppresses angiogenesis by selectively inhibiting against vascular endothelial growth factor. Potent VEGFR inhibitor.
Background
Renal cell carcinoma (RCC) is responsible for 3% of cancer cases and is one of the 10 most common cancers in adults. The average age of diagnosis is between age 65 to 74. Tivozanib, also known as FOTIVDA, is a kinase inhibitor developed to treat adult patients with relapsed or refractory advanced renal cell carcinoma (RCC) after prior failed systemic therapies. It was approved on March 10, 2021 by the FDA. Marketed by Aveo Oncology, tivozanib is a promising therapy for individuals with RCC who have not been treated successfully with other therapies.
Indications
Tivozanib is approved in the USA for the treatment of relapsed or refractory renal cell carcinoma in adult patients who have undergone two or more systemic therapies. In the UK and other countries, is indicated as first line therapy of adults with advanced renal cell carcinoma (RCC) and VEGFR and mTOR pathway inhibitor-na?ve patients after disease progression following one previous treatment with cytokine therapy for advanced disease.
Definition
ChEBI: 1-[2-chloro-4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-3-(5-methyl-3-isoxazolyl)urea is an aromatic ether.
brand name
FotivdaTM
Mechanism of action
Tivozanib is a tyrosine kinase inhibitor that exerts its actions by inhibiting the phosphorylation of vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2 and VEGFR-3 and inhibits other kinases such as c-kit and platelet derived growth factor beta (PDGFR β).
Pharmacokinetics
Tivozanib inhibits growth factor receptors, treating renal cell carcinoma. In mice and rats, tivozanib inhibits tumour angiogenesis, tumour growth, and vascular permeability. Tivozanib was shown to frequently cause hypertension in clinical trials; hypertension must be managed before initiating therapy. Cardiac QT segment prolongation was reported in a tivozanib cardiac safety study, however the reactions were not considered clinically serious. In clinical studies, levels of serum soluble VEGFR2 (sVEGFR2) decreased with time and this effect increased with tivozanib exposure, and sVEGFR2 may serve as a pharmacodynamic marker of VEGFR inhibition.
Side Effects
- diarrhea
- nausea
- vomiting
- fatigue
- loss of appetite
- weight loss
- voice hoarseness
- back pain
- mouth sores
- cough
- shortness of breath
Synthesis
The synthesis of Tivozanib is as follows:
Phenyl chlorocarbonate (601 g) was added dropwise to 3-amino-5-methylisoxazole (377 g), pyridine (1215 g), and N,N-dimethylacetamide (4 L) at 0°C, and the mixture was stirred at 20°C for 2 hr. 4-[(4-Amino-3-chlorophenol)oxy]-6,7-dimethoxyquinoline (847 g) was added to the reaction solution, and the mixture was stirred at 80°C for 5 hr. The reaction solution was cooled to 5°C. Thereafter, methanol (8.5 L) and water (8.5 L) were added thereto, and the mixture was neutralized with an aqueous sodium hydroxide solution. The resultant precipitate was collected by filtration, and the filtered product was slurried in water (8.5 L) for washing. The slurry was filtered, and the filtered product was then dried under the reduced pressure to give Tivozanib (1002 g, yield 86.1%).
Metabolism
Tivozanib is primarily metabolized by CYP3A4. After oral ingestion of a radiolabeled 1.34 mg dose of tivozanib in healthy volunteers, unchanged tivozanib accounted for 90% of the radioactive drug detected in serum.
Properties of Tivozanib
| Melting point: | >202°C (dec.) |
| Boiling point: | 550.4±50.0 °C(Predicted) |
| Density | 1.421 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated) |
| pka | 11.74±0.70(Predicted) |
| form | White powder. |
| color | Pale Beige to Light Brown |
Safety information for Tivozanib
Computed Descriptors for Tivozanib
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 Tivozanib 98% (HPLC) CAS 475108-18-0View Details
Tivozanib 98% (HPLC) CAS 475108-18-0View Details
475108-18-0 -
 Tivozanib >95% CAS 475108-18-0View Details
Tivozanib >95% CAS 475108-18-0View Details
475108-18-0 -
 AV-951 >95% CAS 475108-18-0View Details
AV-951 >95% CAS 475108-18-0View Details
475108-18-0 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4