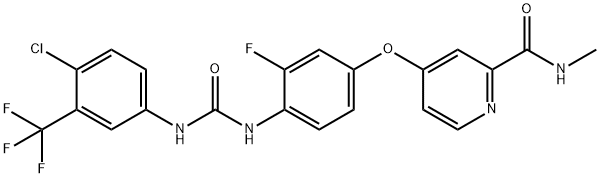
Regorafenib
- CAS NO.:755037-03-7
- Empirical Formula: C21H15ClF4N4O3
- Molecular Weight: 482.82
- MDL number: MFCD16038047
- EINECS: 815-051-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is Regorafenib?
Absorption
Cmax = 2.5 μg/mL; Tmax = 4 hours; AUC = 70.4 μgh/mL; Cmax, steady-state = 3.9 μg/mL; AUC, steady-state = 58.3 μgh/mL; The mean relative bioavailability of tablets compared to an oral solution is 69% to 83%.
Toxicity
The most common adverse reactions (≥20%) are asthenia/fatigue, HFSR, diarrhea, decreased appetite/food intake, hypertension, mucositis, dysphonia, and infection, pain (not otherwise specified), decreased weight, gastrointestinal and abdominal pain, rash, fever, and nausea
Description
In September 2012, the approved regorafenib for the treatment of patients with metastatic colorectal cancer (CRC), especially those for whom standard therapies have failed, including fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFRtherapy. Regorafenib is a multikinase inhibitor with potent inhibitory activity versus VEGFRs and PDFRs. Both of these classes of receptors are expressed on tumour cells and affect proliferation and angiogenesis. Regorafenib inhibited growth in murine xenograft models for colon, breast, renal, lung, melanoma, pancreatic, and ovarian tumours when dosed at 10–30 mg/kg. Regorafenib is a fluorinated analogue of sorafenib, a multikinase inhibitor co-marketed by Bayer and Onyx for treating kidney and liver cancer. The synthesis of regorafenib is accomplished in two steps using commercially available starting materials. 4-Aminophenol is coupled to 4-chloro-N-methyl- 2-pyridinecarboxamide to give 4-(2-(N-methylcarbamoyl)-4-pyridyloxy)aniline. Subsequent treatment with 4-chloro-3-(trifluoromethyl)phenyl isocycanate affords the urea, regorafenib.
Originator
Bayer (Germany)
Characteristics
Class: receptor tyrosine kinase
Treatment: colorectal cancer, GIST, HCC
Elimination half-life = 26-28 h
Protein binding = 99.5%
The Uses of Regorafenib
BAY 73-4506 (Regorafenib) is a multikinase inhibitor with IC50 of 17, 40 and 69 nM c-KIT, VEGFR2, B-Raf.
The Uses of Regorafenib
It inhibits PDGFR tyrosine kinase with IC50=83nM. It is useful for the treatment of inflammation and as an anti-proliferative agent.
The Uses of Regorafenib
Regorafenib (BAY 73-4506) is a multi-target inhibitor for VEGFR1, VEGFR2, VEGFR3, PDGFRβ, Kit, RET and Raf-1 with IC50 of 13 nM/4.2 nM/46 nM, 22 nM, 7 nM, 1.5 nM and 2.5 nM, respectively
What are the applications of Application
Regorafenib is a multi-kinase inhibitor that is orally bioavailable and displays anticancer properties.
Background
Regorafenib is an orally-administered inhibitor of multiple kinases. It is used for the treatment of metastatic colorectal cancer, advanced gastrointestinal stromal tumours, and hepatocellular carcinoma. FDA approved on September 27, 2012. Approved use of Regorafenib was expanded to treat Hepatocellular Carcinoma in April 2017.
Indications
Regorafenib is indicated for the treatment of patients with metastatic colorectal cancer (CRC) who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy. Regorafenib is also indicated for the treatment of patients with locally advanced, unresectable or metastatic gastrointestinal stromal tumour (GIST) who have been previously treated with imatinib mesylate and sunitinib malate. Regorafenib is also indicated for the treatment of patients with hepatocellular carcinoma (HCC) previously treated with sorafenib.
Definition
ChEBI: A pyridinecarboxamide obtained by condensation of 4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]pyridine-2-carboxylic acid with methylamine. Used for for the treatment of metastatic colorectal cancer in patients who have previ usly received chemotherapy, anti-EGFR or anti-VEGF therapy.
brand name
Stivarga
Clinical Use
Treatment of colorectal cancer and gastrointestinal
stromal tumours
Treatment of hepatocellular carcinoma
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: avoid with mefenamic acid.
Antibacterials: concentration reduced by rifampicin
- avoid.
Anticoagulants: increased risk of bleeding with
warfarin.
Antifungals: concentration increased by ketoconazole
- avoid.
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis).
Metabolism
Regorafenib is metabolised by CYP3A4 and UGT1A9. The primary circulating metabolites of regorafenib measured at steady-state in human plasma are M-2 (N-oxide) and M-5 (N-oxide and N-desmethyl), both of them having similar in vitro pharmacological activity and steady-state concentrations as regorafenib. M-2 and M-5 are highly protein-bound (99.8% and 99.95%, respectively). Approximately 90% of the radioactive dose was recovered within 12 days after administration, with about 71% of the dose excreted in faeces (47% as parent compound, 24% as metabolites), and about 19% of the dose excreted in urine as glucuronides. Urinary excretion of glucuronides decreased below 10% under steady-state conditions. Parent compounds found in faeces could be derived from intestinal degradation of glucuronides or reduction of metabolite M-2 (N-oxide), as well as unabsorbed regorafenib.
Biologiacal activity
Regorafenib is an orally bioavailable multikinase inhibitor with anticancer activity. It inhibits RET, C-RAF, VEGFR2, c-Kit, VEGFR1, and PDGFRβ with IC50 values of 1.5, 2.5, 4.2, 7, 13, and 22 nM, respectively. Regorafenib inhibits B-RAF, VEGFR3, FGFR, Tie2 (IC50s = 28, 46, 202, and 311 nM, respectively) and other kinases. In vivo, regorafenib (10 mg/kg) reduces tumour size in the MDA-MB-231 breast and 786-O renal cancer mouse xenograft models. It also reduces tumour microvessel area and inhibits tumour growth in a panel of mouse xenograft models. Formulations containing regorafenib have been used in the treatment of advanced gastrointestinal stromal tumours and metastatic colorectal cancer.
References
[1]. wilhelm sm, dumas j, adnane l, et al. regorafenib (bay 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. int j cancer, 2011, 129(1): 245-255.
[2]. schmieder r, hoffmann j, becker m, et al. regorafenib (bay 73-4506): antitumor and antimetastatic activities in preclinical models of colorectal cancer. int j cancer, 2014, 135(6): 1487-1496.
Properties of Regorafenib
| Melting point: | 206.0 to 210.0 °C |
| Boiling point: | 513.4±50.0 °C(Predicted) |
| Density | 1.491±0.06 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 12.04±0.70(Predicted) |
| form | White powder. |
| color | Pale Pink to Light Pink |
| CAS DataBase Reference | 755037-03-7 |
Safety information for Regorafenib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Regorafenib
Regorafenib manufacturer
ALS India Life Sciences Pvt. Ltd
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 755037-03-7 REGORAFENIB 99%View Details
755037-03-7 REGORAFENIB 99%View Details
755037-03-7 -
 755037-03-7 98%View Details
755037-03-7 98%View Details
755037-03-7 -
 755037-03-7 Regorafenib 98%View Details
755037-03-7 Regorafenib 98%View Details
755037-03-7 -
 755037-03-7 95-99%View Details
755037-03-7 95-99%View Details
755037-03-7 -
 Regorafenib >95% CAS 755037-03-7View Details
Regorafenib >95% CAS 755037-03-7View Details
755037-03-7 -
 Regorafenib CAS 755037-03-7View Details
Regorafenib CAS 755037-03-7View Details
755037-03-7 -
 Regorafenib 95% CAS 755037-03-7View Details
Regorafenib 95% CAS 755037-03-7View Details
755037-03-7 -
 Regorafenib 755037-03-7 95.00%View Details
Regorafenib 755037-03-7 95.00%View Details
755037-03-7
