Testosterone cypionate
Synonym(s):4-Androsten-17β-ol-3-one 17-cypionate;Depotestosterone;Depovirin;Testosterone 17β-cyclopentanepropionate;Testosterone 17β-cypionate
- CAS NO.:58-20-8
- Empirical Formula: C27H40O3
- Molecular Weight: 412.6
- MDL number: MFCD00053944
- EINECS: 200-368-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-18 18:20:25
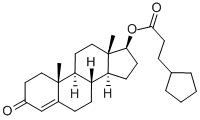
What is Testosterone cypionate?
Absorption
Testosterone cypionate is an esterified anabolic which allows it to present a greater degree of solubility in fats and thus, the release and absorption occur in a slow rate compare to homologous molecules. Intramuscular administration of 200 mg of testosterone cypionate produced a mean supratherapeutic Cmax of 1122 ng/dl which occurred 4-5 days post-injection. After the fifth day, the levels of testosterone cypionate in plasma went down reaching an average of 400 ng/dl.
Toxicity
Preclinical studies with testosterone implants induced cervical-uterine tumors in mice which metastasized in some cases. Some reports indicate that administration of testosterone cypionate in females can augment the susceptibility to hepatoma as well as increase the number of tumors. Clinical studies have reported cases of hepatocellular carcinoma in long-term high-dose therapy.
Chemical properties
Testosterone cypionate is a white or creamy white crystalline powder, odorless or nearly so and stable in air. It is insoluble in water, freely soluble in alcohol, chloroform, dioxane, ether, and soluble in vegetable oils.
Originator
Depo-Testosterone,Upjohn,US,1951
The Uses of Testosterone cypionate
Testosterone Cypionate is an anabolic steroid with androgenic properties. Testosterone Cypionate is used for testosterone therapy in males with hypogonadism. Controlled Substance.
Background
Testosterone cypionate is a synthetic derivative of testosterone in the form of an oil-soluble 17 (beta)-cyclopentylpropionate ester. Its benefit compared to other testosterone derivatives is the slow rate of release after injection and longer half-life. It was developed by the company Pharmacia and Upjohn and FDA approved on July 25, 1979.
Indications
Testosterone cypionate is used in males that present conditions derived from a deficiency or absence of endogenous testosterone. These conditions are 1) primary hypogonadism, defined as the testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome or orchidectomy; and 2) hypogonadotropic hypogonadism characterized by idiopathic gonadotropin, LHRH deficiency or pituitary-hypothalamic injury from tumors, trauma or radiation.
Definition
ChEBI: Testosterone cypionate is a sterol ester. It is functionally related to a 3-cyclopentylpropionic acid and a testosterone.
Manufacturing Process
1 g of crude 3-enol-ethyl ether of testosterone dissolved in 3 cc of pyridine is treated with 2 cc of β-cyclopentylpropionic anhydride (obtained from the β- cyclopentylpropionic acid and acetic anhydride: boiling point 180°C/2 mm Hg). After standing at room temperature overnight the mixture is diluted with water and extracted with ether, the ethereal layer, washed with water to neutrality and dried, is evaporated by vacuum. The oily residue is taken up in petroleum ether and filtered through a layer of aluminum oxide, which is afterwards washed with a further amount of petroleum ether. The solution so filtered and purified is evaporated to dryness; the crystalline residue is recrystallized from a small amount of methanol containing a trace of pyridine: about 1 g of 3-enol-ethyl-ether of the β-cyclopentyl propionate of testosterone, melting point 86°C to 88°C, is so obtained (by further recrystallization melting point 90°C to 91°C). This product (that may be employed either in the crystalline state, or in the oily one, that is, before the purification by filtration through aluminum oxide) by treatment with a small amount of hydrochloric acid in acetone solution yields the β-cyclopentyl propionate of testosterone, melting point 99°C to 101°C (recrystallized from methanol).
Therapeutic Function
Androgen
Pharmacokinetics
Administration of ester derivatives of testosterone as testosterone cypionate generates an increase in serum testosterone to levels reaching 400% from the baseline within 24 hours of administration. These androgen levels remain elevated for 3-5 days after initial administration. The continuous variation in plasma testosterone after intramuscular administration of testosterone cypionate results in fluctuations in mood and libido as well as some local inflammation.
Veterinary Drugs and Treatments
The use of injectable esters of testosterone in veterinary medicine is
limited primarily to its use in dogs (and perhaps cats) for the treatment
of testosterone-responsive urinary incontinence in neutered
males. Testosterone has been used to treat a rare form of dermatitis
(exhibited by bilateral alopecia) in neutered male dogs. These drugs
are also used in bovine medicine to produce an estrus-detector
(teaser) animal in cull cows, heifers, and steers.
The effectiveness of testosterone to increase libido, treat hypogonadism,
aspermia, and infertility in domestic animals has been disappointing.
Metabolism
To start its activity, testosterone cypionate has to be processed by enzymes in the bloodstream. These enzymes will break the bond between the cypionate ester moiety and the testosterone. Once separated, testosterone is metabolized to 17-keto steroids through two different pathways. The major active metabolites are estradiol and dihydrotestosterone (DHT). Testosterone is metabolized to DHT by steroid 5α-reductase in skin, liver and urogenital tract. In reproductive tissues DHT is further metabolized to androstanediol.
References
[1] BEHRE H, NIESCHLAG E. Testosterone: Testosterone preparations for clinical use in males[C]. 2012: 0. DOI:10.1017/CBO9781139003353.016.
[2] H M BEHRE. Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: phase I studies.[J]. European Journal of Endocrinology, 1999, 140 5: 414-419. DOI:10.1530/eje.0.1400414.
[3] V. DUMESTRE-TOULET. Hair analysis of seven bodybuilders for anabolic steroids, ephedrine, and clenbuterol.[J]. Journal of forensic sciences, 1900, 47 1 1: 211-214. DOI:10.1520/JFS15228J.
[4] YOUWEN YOU. Simultaneous separation and determination of 16 testosterone and nandrolone esters in equine plasma using ultra high performance liquid chromatography-tandem mass spectrometry for doping control.[J]. Journal of Chromatography A, 2011, 1218 26: 3982-3993. DOI:10.1016/j.chroma.2011.04.087.
[5] J. SHOSKES M. S M Wilson. Pharmacology of testosterone replacement therapy preparations[J]. Translational andrology and urology, 2016, 5 1: 834-843. DOI:10.21037/tau.2016.07.10.
[6] https://www.pfizermedicalinformation.com/depo-testosterone/description
Properties of Testosterone cypionate
| Melting point: | 101-102° |
| Boiling point: | 492.01°C (rough estimate) |
| alpha | D25 +87° (CHCl3) |
| Density | 0.9795 (rough estimate) |
| refractive index | 1.5460 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMF: 25 mg/mL; DMSO: 5 mg/mL; Ethanol: 15 mg/mL; Ethanol:PBS (pH 7.2)(1:2): 0.3 mg/mL |
| form | A crystalline solid |
| CAS DataBase Reference | 58-20-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Testosterone cypionate(58-20-8) |
Safety information for Testosterone cypionate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H351:Carcinogenicity H361:Reproductive toxicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Testosterone cypionate
| InChIKey | HPFVBGJFAYZEBE-ZLQWOROUSA-N |
| SMILES | [C@@]12([H])CCC3=CC(=O)CC[C@]3(C)[C@@]1([H])CC[C@]1(C)[C@H](CC[C@@]21[H])OC(=O)CCC1CCCC1 |&1:0,10,12,16,18,21,r| |
Testosterone cypionate manufacturer
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran
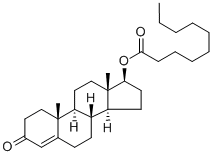
You may like
-
 58-20-8 Testosterone 17Beta-cypionate 98%View Details
58-20-8 Testosterone 17Beta-cypionate 98%View Details
58-20-8 -
 58-20-8 98%View Details
58-20-8 98%View Details
58-20-8 -
 Testosterone 17Beta-cypionate 98%View Details
Testosterone 17Beta-cypionate 98%View Details
58-20-8 -
 Testosterone 17Beta-cypionate 58-20-8 98%View Details
Testosterone 17Beta-cypionate 58-20-8 98%View Details
58-20-8 -
 58-20-8 Testosterone 17Beta-cypionate 98%View Details
58-20-8 Testosterone 17Beta-cypionate 98%View Details
58-20-8 -
 Testosterone 17Beta-cypionate 98%View Details
Testosterone 17Beta-cypionate 98%View Details
58-20-8 -
 58-20-8 99%View Details
58-20-8 99%View Details
58-20-8 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8
