Cyclopentene
- CAS NO.:142-29-0
- Empirical Formula: C5H8
- Molecular Weight: 68.12
- MDL number: MFCD00001394
- EINECS: 205-532-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-07 18:45:43
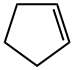
What is Cyclopentene?
Chemical properties
Cyclopentene is a highly flammable liquid with a low flash point. It reacts readily with oxidizing agents.
Physical properties
Clear, colorless, watery, very flammable liquid with a characteristic sweet, petroleum-like odor.
The Uses of Cyclopentene
Cyclopentene is a cycloalkene that is cyclopentane having one endocyclic double bond. Vapors heavier than air. Inhalation of high concentrations may be narcotic. Used to make rubber and plastics. Neopentyl phosphine ligand catalyzed Heck coupling of cyclopentene has been reported. Mechanism of reaction of ground state oxygen atom with cyclopentene has been investigated. Homopolymerization of cyclopentene has been reported. Photocatalytic oxidation of cyclopentene over various titanium(IV) oxide catalyst has been reported. Cyclopentene was used to investigate the [2+2] cycloaddition of diamond (001) surfaces with alkene.
The Uses of Cyclopentene
Cyclopentene is used in organic synthesis for cross-linking resin. It is used as an intermediate in Industries such as Agrochemical, Dyestuff, Pharmaceutical, syntheses material, Chemical. It is used as a monomer for synthesis of plastics and rubber. It is also used for the synthesis of various chemicals, such as 1,1,2-Trimethylcyclohexane.
Definition
ChEBI: Cyclopentene is a cycloalkene that is cyclopentane having one endocyclic double bond.
Preparation
Cyclopentene is synthesized by selective hydrogenation of cyclopentadiene or by dehydration of cyclopentanol. It is produced industrially in large amounts by steam cracking of naphtha. Cyclopentene is present in coal tar, cigarette smoke, and automobile emissions.
General Description
Cyclopentene appears as a colorless liquid. Less dense than water and insoluble in water. Flash point below 0°F. Vapors heavier than air. Inhalation of high concentrations may be narcotic. Used to make rubber and plastics.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Cyclopentene may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release hydrogen gas. In the presence of various catalysts (such as acids) or initiators, may undergo exothermic addition polymerization reactions.
Health Hazard
May be harmful by inhalation, ingestion, or skin absorption. May cause eye and skin irritation.
Fire Hazard
Special Hazards of Combustion Products: Vapor may travel considerable distance to a source of ignition and flashback. Explosion may occur under fire condition.
Safety Profile
Moderately toxic by ingestion and skin contact. A very dangerous fire hazard when exposed to flame or heat; can react with oxidning materials. Keep away from heat and open flame. To fight fire, use foam, CO2, dry chemical.
Source
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rate of cyclopentene was 7.8 mg/kg of pine burned. Emission rates of cyclopentene were not
measured during the combustion of oak and eucalyptus.
California Phase II reformulated gasoline contained cyclopentene at a concentration of 1,120
mg/kg. Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without
catalytic converters were 480 and 31,700 μg/km, respectively (Schauer et al., 2002).
Given that cyclopentene is prepared from cyclohepentanol, the latter may be present as an
impurity.
Environmental Fate
Biological. Cyclopentene may be oxidized by microbes to cyclopentanol, which may oxidize to
cyclopentanone (Dugan, 1972).
Photolytic. The following rate constants were reported for the reaction of cyclopentene with OH
radicals in the atmosphere: 6.39 x 10-11 cm3/molecule?sec (Atkinson et al., 1983), 4.99 x 10-11
cm3/molecule?sec at 298 K (Rogers, 1989), 4.0 x 10-10 cm3/molecule?sec (Atkinson, 1990) and
6.70 x 10-11 cm3/molecule?sec (Sablji? and Güsten, 1990); with ozone in the atmosphere: 8.13 x
10-16 at 298 K (Japar et al., 1974) and 9.69 x 10-16 cm3/molecule?sec at 294 K (Adeniji et al.,
1981); with NO3 in the atmosphere: 4.6 x 10-13 cm3/molecule?sec at 298 K (Atkinson, 1990) and
5.81 x 10-13 cm3/molecule?sec at 298 K (Sablji? and Güsten, 1990).
Chemical/Physical. Gaseous products formed from the reaction of cyclopentene with ozone
were (% yield): formic acid, carbon monoxide, carbon dioxide, ethylene ,
formaldehyde, and butanal. Particulate products identified include succinic acid,
glutaraldehyde, 5-oxopentanoic acid, and glutaric acid (Hatakeyama et al., 1987).
At elevated temperatures, rupture of the C-C bond occurs forming molecular hydrogen and
cyclopentadiene (95% yield) as the principal products (Rice and Murphy, 1942).
Purification Methods
Free cyclopentene from hydroperoxide by refluxing with cupric stearate. Fractionally distil it from Na. It can be chromatographed on a Dowex 710-Chromosorb W GLC column. Methods for cyclohexene should be applicable here. Also, it has been washed with 1M NaOH solution followed by water. It was dried over anhydrous Na2SO4, distilled over powdered NaOH under nitrogen, and passed through neutral alumina before use [Woon et al. J Am Chem Soc 108 7990 1986]. It was distilled in a dry nitrogen atmosphere from powdered fused NaOH through a Vigreux column (p 11), and then passed through activated neutral alumina before use [Wong et al. J Am Chem Soc 109 3428 1987]. [Beilstein 5 IV 209.]
Toxicity evaluation
Acute Toxicity. The oral LD50 in the rat is
1656 mL/kg, and the dermal LD50 in the rabbit is
1231 mL/kg. Inhalation of the concentrated vapor was lethal
to rats in 5min, and a 4 h exposure to 16,000 ppm was lethal
to four of six rats.
Chronic and Subchronic Toxicity. Chronic
exposure of rats to 112–1139 ppm for 12 weeks showed
no effects, whereas 8110 ppm for 6 h/day, 5 days/week for
3 weeks resulted in decreased body weight gains of female
rats.
Human Experience
General Information. Short-term exposure of
cyclopentene to humans revealed a tolerable level of only
10–15 ppm.
Properties of Cyclopentene
| Melting point: | −135 °C(lit.) |
| Boiling point: | 44-46 °C(lit.) |
| Density | 0.771 g/mL at 25 °C(lit.) |
| vapor pressure | 20.89 psi ( 55 °C) |
| refractive index | n |
| Flash point: | <−30 °F |
| storage temp. | 0-6°C
|
| solubility | water: soluble0.535g/L at 25°C |
| form | Liquid |
| color | Colorless |
| Specific Gravity | 0.771 |
| Water Solubility | immiscible |
| Sensitive | Air Sensitive |
| BRN | 635707 |
| Henry's Law Constant | 6.3 x 10-2 atm?m3/mol at 25 °C (Hine and Mookerjee, 1975) |
| Stability: | Stable. Highly flammable. Incompatible with strong oxidizing agents. Store cold. |
| CAS DataBase Reference | 142-29-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Cyclopentene(142-29-0) |
| EPA Substance Registry System | Cyclopentene (142-29-0) |
Safety information for Cyclopentene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Cyclopentene
| InChIKey | LPIQUOYDBNQMRZ-UHFFFAOYSA-N |
Cyclopentene manufacturer
Navone Specialties (OPC) Pvt Ltd
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


![Cyclopenta[b]pyridine](https://img.chemicalbook.in/CAS/GIF/533-37-9.gif)
You may like
-
 Cyclopentene 142-29-0 99%View Details
Cyclopentene 142-29-0 99%View Details
142-29-0 -
 Cyclopentene CAS 142-29-0View Details
Cyclopentene CAS 142-29-0View Details
142-29-0 -
 Cyclopentene CAS 142-29-0View Details
Cyclopentene CAS 142-29-0View Details
142-29-0 -
 Cyclopentene CAS 142-29-0View Details
Cyclopentene CAS 142-29-0View Details
142-29-0 -
 Cyclopentene CAS 142-29-0View Details
Cyclopentene CAS 142-29-0View Details
142-29-0 -
 Cyclopentene CAS 142-29-0View Details
Cyclopentene CAS 142-29-0View Details
142-29-0 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4
