Testosterone acetate
Synonym(s):17β-Acetoxy-4-androsten-3-one solution;17β-Hydroxy-4-androsten-3-one 17-acetate solution;4-Androsten-17β-ol-3-one 17-acetate solution;Deposteron solution
- CAS NO.:1045-69-8
- Empirical Formula: C21H30O3
- Molecular Weight: 330.46
- MDL number: MFCD00021154
- EINECS: 213-876-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-20 19:16:41
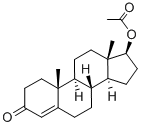
What is Testosterone acetate?
Description
Testosterone acetate is an analytical reference standard categorized as an anabolic androgenic steroid. It is an ester of the naturally occurring androgen, testosterone. Testosterone acetate is regulated as a Schedule III compound in the United States. This product is intended for research and forensic applications.
Chemical properties
White Solid
The Uses of Testosterone acetate
Testosterone Acetate is a Controlled substance that is less popular due to Acetate solution, which is aqueous solution, instead of oil solution as other esterified versions of testosterone that is offering much longer half life. It does not have any medical uses when it comes with Acetate ester. However, taken in consideration that it works in the same way as any other esterified testosterone, it can be used for the same purposes as the esterified testosterones.
Definition
ChEBI: Testosterone acetate is an androstanoid that is the acetate derivative of testosterone. It has a role as a human metabolite. It is a sterol ester, an androstanoid and a 3-oxo-Delta(4) steroid. It is functionally related to a testosterone.
Synthesis
A solution of 4.23 g (14.7 mmol) of testosterone in 8 ml of pyridine dried over potassium hydroxide overnight (5 g of KOH to 250 ml of pyridine) is prepared in a 250-ml flask equipped with magnetic stirring. The solution is cooled to 0° C. with a water/ice mixture. 4 ml of acetic anhydride is added to it dropwise. The reaction mixture is left to return to room temperature, and the solution is stirred overnight at RT. After the starting product is no longer found in analytical TLC, 30 ml of distilled water and 30 ml of ethyl acetate are poured into the flask, and the mixture is stirred. The phases are separated with a separating funnel, and the aqueous phase is extracted with 3×20 ml of ethyl acetate. The organic phases are combined, and the solution obtained is washed with 20 nil of distilled water, dried over anhydrous magnesium sulfate, and evaporated in a rotary evaporator. The product thus obtained is dried under reduced pressure using a vacuum pump for 4 hours to remove all the solvents. 4.8 g (yield=99.0%) of testosterone acetate is obtained.
General Description
Testosterone acetate is an androgen ester and an intermediate in the synthesis of testosterone from progesterone, isolated from microorganisms.
Properties of Testosterone acetate
| Melting point: | 139-141 °C |
| Boiling point: | 427.89°C (rough estimate) |
| Density | 1.0234 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| Flash point: | 2℃ |
| storage temp. | Controlled Substance, -20°C Freezer |
| solubility | Acetonitrile: 1 mg/ml; Ethanol: 1 mg/ml; Methanol: 1 mg/ml |
| form | A crystalline solid |
| Water Solubility | 2.35mg/L(25 ºC) |
| Merck | 13,9255 |
| InChI | InChI=1/C21H30O3/c1-13(22)24-19-7-6-17-16-5-4-14-12-15(23)8-10-20(14,2)18(16)9-11-21(17,19)3/h12,16-19H,4-11H2,1-3H3/t16-,17-,18-,19-,20-,21-/s3 |
| CAS DataBase Reference | 1045-69-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Androst-4-en-3-one, 17-(acetyloxy)-, (17«beta»)-(1045-69-8) |
Safety information for Testosterone acetate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Testosterone acetate
| InChIKey | DJPZSBANTAQNFN-PXQJOHHUSA-N |
| SMILES | [C@]12([H])CC[C@]3(C)[C@H](CC[C@@]3([H])[C@]1([H])CCC1=CC(=O)CC[C@]21C)OC(=O)C |&1:0,4,6,9,11,21,r| |
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 1045-69-8 Testosterone EP Impurity E 98%View Details
1045-69-8 Testosterone EP Impurity E 98%View Details
1045-69-8 -
 1045-69-8 98%View Details
1045-69-8 98%View Details
1045-69-8 -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
