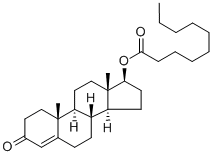
Testosterone enanthate
Synonym(s):(17β)-17-[(1-Oxoheptyl)oxy]androst-4-en-3-one;17β-Hydroxy-4-androsten-3-one 17-enanthate;4-Androsten-17β-ol-3-one 17-enanthate;Testosterone 17β-heptanoate;Testosterone enanthate
- CAS NO.:315-37-7
- Empirical Formula: C26H40O3
- Molecular Weight: 400.59
- MDL number: MFCD00055890
- EINECS: 206-253-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-19 18:47:05
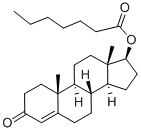
What is Testosterone enanthate?
Absorption
The pharmacokinetic profile of testosterone enanthate was studied in a regime of multiple dosing and the testosterone level was reported to present a Cmax above 1200 ng/dl after 24 hours of the last dose. The concentration decreased sequentially until it reached 600 ng/dl after one week. The pharmacokinetic profile of testosterone enanthate presented differences depending on the administered dose in which the tmax was shifted to a range of 36-48 hours. The plasma testosterone level plateaued below the therapeutic range after 3-4 weeks. This reports showed that the different formulation of testosterone enanthate and testosterone cypionate generates a different profile and thus, they are not therapeutically equivalent.
Toxicity
Testosterone enanthate has been tested in preclinical carcinogenesis trials. In this studies, it is suggested that the exposure to this drug may increase the susceptibility to hematoma as well as the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver.
Testosterone enanthate is not indicated for use in females and is contraindicated in pregnant women. Testosterone is teratogenic and may cause fetal harm when administered to a pregnant woman based on data from animal studies and its mechanism of action.
During treatment with large doses of exogenous androgens, including testosterone enanthate, spermatogenesis may be suppressed through feedback inhibition of the hypothalamic-pituitary-testicular axis . Reduced fertility is observed in some men taking testosterone replacement therapy and the impact on fertility may be irreversible .
Safety and effectiveness of testosterone enanthate in pediatric patients less than 18 years old have not been established . Improper use may result in the acceleration of bone age and premature closure of epiphyses .
Geriatric patients treated with androgens may also be at risk for worsening of signs and symptoms of Benign Prostatic Hyperplasia .
Description
Testosterone enanthate (Item No. 22546) is an analytical reference standard categorized as an anabolic androgenic steroid. It is an ester of the naturally occurring androgen, testosterone (Item Nos. 15645 | ISO60154) with a longer half-life. Formulations containing testosterone enanthate have been tested for use in hypogonadism and as a potential male contraceptive. Formulations containing it have been associated with higher levels of total cholesterol, low density lipoproteins, and triglycerides and lower levels of high density lipoproteins. Anabolic steroids, including testosterone enanthate, have been used to enhance physical performance in racehorses and athletes, and methods to detect steroids, their derivatives, and their metabolites have been developed. This product is intended for research and forensic applications.
Chemical properties
White or yellowish-white, crystalline powder.
Originator
Delatestryl,Squibb,US,1954
The Uses of Testosterone enanthate
Testosterone Enanthate is a derivative of testosterone (T155000), the principal hormone of the testes, produced by the interstitial cells.
Indications
Testosterone enanthate in males is indicated as a replacement therapy in conditions associated with a deficiency or absence of endogenous testosterone. Some of the treated conditions are 1) primary hypogonadism, defined as testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome or orchidectomy; 2) hypogonadotropic hypogonadism due to an idiopathic gonadotropin or luteinizing hormone-releasing hormone deficiency or due to a pituitary-hypothalamic injury from tumors, trauma or radiation, in this case it is important to accompany the treatment with adrenal cortical and thyroid hormone replacement therapy; 3) to stimulate puberty in patients with delayed puberty not secondary to a pathological disorder. If the conditions 1 and 2 occur prior to puberty, the androgen replacement therapy will be needed during adolescent years for the development of secondary sexual characteristics and prolonged androgen treatment might be needed it to maintain sexual characteristics after puberty.
In females, testosterone enanthate is indicated to be used secondarily in presence of advanced inoperable metastatic mammary cancer in women who are from one to five years postmenopausal. It has also been used in premenopausal women with breast cancer who have benefited from oophorectomy and are considered to have a hormone-responsive tumor.
Testosterone enanthate injections that are currently formulated for subcutaneous use are specifically indicated only for primary hypogonadism and hypogonadotropic hypogonadism . The use of such formulations is limited because the safety and efficacy of these subcutaneous products in adult males with late-onset hypogonadism and males less than 18 years old have not yet been established . Moreover, subcutaneously administered testosterone enanthate is indicated only for the treatment of men with hypogonadal conditions associated with structural or genetic etiologies, considering the medication could cause blood pressure increases that can raise the risk of major adverse cardiovascular events like non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death .
Background
Testosterone enanthate is an esterified variant of testosterone that comes as an injectable compound with a slow-release rate. This slow release is achieved by the presence of the enanthate ester functional group attached to the testosterone molecule. This testosterone derivative was first approved on December 24, 1953.
In 2017, about 6.5 million retail prescriptions for testosterone therapy were filled . The majority of the prescriptions written were for injectable (66%) and topical (32%) testosterone products. As recent as 1 October 2018, the US FDA approved Antares Pharma Inc.'s Xyosted - a subcutaneous testosterone enanthate product for once-weekly, at-home self-administration with an easy-to-use, single dose, disposable autoinjector . As the first subcutaneous autoinjector product designed for testosterone replacement therapy, this innovative formulation removes transfer concerns commonly associated with testosterone gels and potentially reduces the need for in-office/in-clinic injection procedures that may inconvenience patients with frequent visits to the clinic .
Definition
ChEBI: Testosterone enanthate is a heptanoate ester and a sterol ester. It has a role as an androgen. It is functionally related to a testosterone.
Manufacturing Process
A mixture of testosterone, pyridine and oenanthic acid anhydride is heated for 1 1/2 hours to 125°C. The cooled reaction mixture is decomposed with water while stirring and cooling. After prolonged standing at a temperature below room temperature, the whole is extracted with ether and the ethereal solution is washed consecutively with dilute sulfuric acid, water, 5% sodium hydroxide solution, and again with water. The crude ester remaining on evaporation of the dried ether solution, after recrystallization from pentane, melts at 36° to 37.5°C.
Therapeutic Function
Androgen
Pharmacokinetics
Administration of ester derivatives of testosterone as testosterone enanthate generates an increase in serum testosterone to levels reaching 400% from the baseline within 24 hours of administration. These androgen levels remain elevated for 3-5 days after initial administration. Continuous administration of testosterone enanthate shows a significant suppression of dihydrotestosterone, serum PSA, HDL and FSH, as well as a slight increase in serum estradiol. The levels of dihydrotestosterone and FSH can remain suppressed even 14 days after treatment termination. There are no changes in mood and sexual activity by the presence of testosterone enanthate.
Metabolism
To start its activity, testosterone enanthate has to be processed by enzymes in the bloodstream. These enzymes will catalyze the molecule at the ester location of the moiety. Once processed in this manner, the testosterone enanthate molecule is metabolized to various 17-keto steroids through two different pathways. Subsequently, the major active metabolites are estradiol and DHTd. Testosterone is metabolized to DHT by steroid 5α-reductase in skin, liver and urogenital tract. In reproductive tissues DHT is further metabolized to androstanediol.
Properties of Testosterone enanthate
| Melting point: | 34-39°C |
| alpha | 77 º |
| Boiling point: | 463.22°C (rough estimate) |
| Density | 1.0562 (rough estimate) |
| refractive index | 1.4700 (estimate) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, very soluble in anhydrous ethanol, freely soluble in fatty oils. |
| form | neat |
| InChI | InChI=1/C26H40O3/c1-4-5-6-7-8-24(28)29-23-12-11-21-20-10-9-18-17-19(27)13-15-25(18,2)22(20)14-16-26(21,23)3/h17,20-23H,4-16H2,1-3H3/t20-,21-,22-,23-,25-,26-/s3 |
| CAS DataBase Reference | 315-37-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Testosterone enanthate(315-37-7) |
Safety information for Testosterone enanthate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H350:Carcinogenicity H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Testosterone enanthate
| InChIKey | VOCBWIIFXDYGNZ-IXKNJLPQSA-N |
| SMILES | [C@@]12([H])CCC3=CC(=O)CC[C@]3(C)[C@@]1([H])CC[C@]1(C)[C@H](CC[C@@]21[H])OC(=O)CCCCCC |&1:0,10,12,16,18,21,r| |
Testosterone enanthate manufacturer
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran


You may like
-
 315-37-7 Testosterone enanthate 98%View Details
315-37-7 Testosterone enanthate 98%View Details
315-37-7 -
 315-37-7 98%View Details
315-37-7 98%View Details
315-37-7 -
 Testosterone enanthate 98%View Details
Testosterone enanthate 98%View Details
315-37-7 -
 Testosterone enanthate 315-37-7 98%View Details
Testosterone enanthate 315-37-7 98%View Details
315-37-7 -
 Testosterone enanthate 315-37-7 98%View Details
Testosterone enanthate 315-37-7 98%View Details
315-37-7 -
 Testosterone enanthate 99%View Details
Testosterone enanthate 99%View Details
315-37-7 -
 Testosterone Enanthate extrapure CAS 315-37-7View Details
Testosterone Enanthate extrapure CAS 315-37-7View Details
315-37-7 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8
