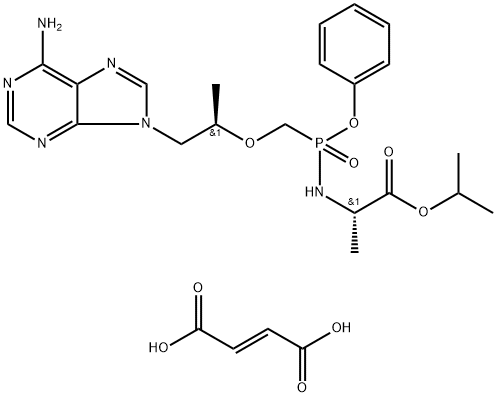
Tenofovir disoproxil
- CAS NO.:201341-05-1
- Empirical Formula: C19H30N5O10P
- Molecular Weight: 519.44
- MDL number: MFCD00952920
- EINECS: 606-442-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
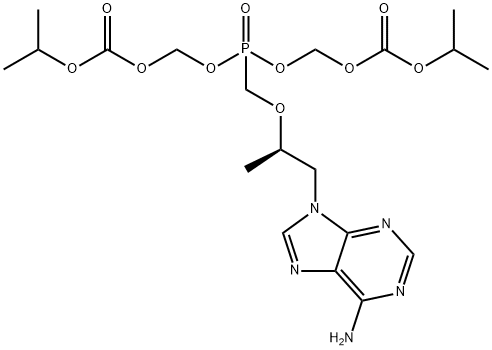
What is Tenofovir disoproxil?
Absorption
After oral administration of tenofovir disoproxil to patients with HIV infection, tenofovir disoproxil is quickly absorbed and metabolized to tenofovir .
Administration of tenofovir disoproxil 300 mg tablets after a high-fat meal increases the oral bioavailability of this drug, as demonstrated by an increase in tenofovir AUC0-∞ of about 40% as well as an increase in Cmax of about 14%. On the contrary, the administration of tenofovir disoproxil with a light meal did not exert a relevant effect on the pharmacokinetics of tenofovir when compared to administration under fasting conditions. The presence of ingested food slows the time to tenofovir Cmax by approximately 1 hour. Cmax and AUC of tenofovir are 0.33 ± 0.12 μg/mL and 3.32 ± 1.37 μg?hr/mL after several doses of tenofovir disoproxil 300 mg once daily in the fed state when meal content is not controlled .
Toxicity
A note on breastfeeding
The Centers for Disease Control and Prevention recommend that HIV-1-infected mothers not breast-feed their infants to prevent postnatal transmission of HIV-1. Mothers should be advised not to breast-feed if they are receiving tenofovir disoproxil .
Carcinogenesis
Long-term oral carcinogenicity studies of tenofovir disoproxil fumarate in mice and rats were performed at exposures up to approximately 16 times (mice) and 5 times (rats) those observed in humans at the therapeutic dose for HIV-1 infection. At the higher dose in female mice, liver adenomas were increased at exposures 16 times that in humans. In rats, the study was negative for carcinogenic findings at exposures up to 5 times that observed in humans at the therapeutic dose .
Pregnancy
This drug is considered a pregnancy Category B drug. Reproduction studies have been performed in rats and rabbits at doses up to 14 and 19 times the recommended human dose based on body surface area comparisons and revealed no evidence of impaired fertility or harm to the fetus due to tenofovir. There are, however, no adequate and well-controlled studies in pregnant women.
Because animal reproduction studies are not consistently reflective of human effects, tenofovir disoproxil should be used during pregnancy only if clearly required. To monitor fetal outcomes of pregnant women taking tenofovir disoproxil, an Antiretroviral Pregnancy Registry has been formed. Healthcare providers are encouraged and advised to register patients by calling the number listed on the FDA label for tenofovir disoproxil .
Mutagenesis
Tenofovir disoproxil fumarate was mutagenic in the in vitro mouse lymphoma assay and negative for mutagenesis in an in vitro bacterial mutagenicity test (Ames test). In an in vivo mouse micronucleus assay, tenofovir disoproxil fumarate was negative when administered to male mice.
Impairment of Fertility
There were no observed effects on fertility, mating performance or early embryonic development when tenofovir disoproxil fumarate was given to male rats at a dose comparable to 10 times the human dose based on body surface area comparisons for 28 days before mating and to female rats for 15 days before mating through day seven of gestation. There was, however, changes in the estrous cycle in female rats .
Description
Tenofovir disoproxil is a prodrug in a manner similar to that of adefovir dipivoxil. In both cases, the phosphate esters are removed through the action of plasma esterase, leading in this case to tenofovir, which differs from adefovir by the presence of the indicated methyl group. Tenofovir disoproxil exhibits good bioavailability (25%), which is improved in the presence of food (35%). The drug is approved for the treatment of HIV infections in adult patients. Tenofovir diphosphate is an HIV RT inhibitor. The active form of tenofovir is the tenofovir diphosphate, which competes with dATP for incorporation into viral DNA, and when incorporated, tenofovir diphosphate results in premature termination of DNA growth and inhibition of DNA polymerase. Tenofovir disoproxil is indicated for treatment-experienced patients with HIV-1. The drug also appears to be effective in treatment-naive patients, but initial approval is for treatment-experienced patients. The drug is administered as one tablet taken once daily. It is recommended that the drug be combined with other RT inhibitors or HIV PIs, which results in additive or synergistic activity.
The Uses of Tenofovir disoproxil
Tenofovir Disoproxil is a therapeutic option for nucleos(t)ide analog (NA)-experienced chronic hepatitis B (CHB) patients infected with hepatitis B virus (HBV). Also, it is an intermediate used in the synthesis of Tenofovir Disoproxil Dimer (T018515), which is a Tenofovir Disoproxil impurity.
Indications
Tenofovir disoproxil is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in patients ≥2 years old and weighing ≥10 kg. It is also indicated for the treatment of chronic hepatitis B in patients ≥2 years old and weighing ≥10 kg.
Tenofovir disoproxil is also an ingredient in several combination products, all of which are indicated either alone or in combination with other antiretrovirals for the treatment of HIV-1 infection.
In addition, tenofovir disoproxil is available in combination with emtricitabine (under the brand name Truvada) for use as pre-exposure prophylaxis (PrEP) in at-risk adults and adolescents weighing ≥ 35kg to reduce the risk of sexually-acquired HIV-1 infection.
Background
Tenofovir disoproxil fumarate (a prodrug of tenofovir), marketed by Gilead Sciences under the trade name Viread, belongs to a class of antiretroviral drugs known as nucleotide analogue reverse transcriptase inhibitors (nRTIs). This drug is prescribed in combination with other drugs for the management of HIV infection as well as for Hepatitis B therapy. Tenofovir disoproxil was initially approved in 2001 .
Definition
ChEBI: An organic phosphonate that is the disoproxil ester of tenofovir. A prodrug for tenofovir, an HIV-1 reverse transcriptase inhibitor, tenofovir disoproxil is used as the fumaric acid salt in combination therapy for the treatment of HIV infection.
General Description
Tenofovir disoproxil is a prodrug analogously with abacavir.Plasma and tissue esterases cleave the phosphateprotecting groups, releasing the active drug. The bioavailabilityof tenofovir is about 35% when administered withfood. The drug is approved by the Food and DrugAdministration (FDA) for the treatment of HIV infectionsin adult patients. Recommendations are for the drug to beadministered with other RT inhibitors or PIs to achievesynergism.
Pharmacokinetics
This drug prevents viral DNA chain elongation through inhibition of enzymes necessary for host cell infection viral replication in HIV-1 and Hepatitis B infections , .
In vitro effects
The antiviral activity of tenofovir against in laboratory and clinical isolates of HIV-1 was studied in lymphoblastoid cell lines, primary monocyte/macrophage cells, in addition to peripheral blood lymphocytes. The EC50 (50% effective concentration) values of tenofovir against HIV-1 virus ranged between 0.04 μM to 8.5 μM.
Combination of tenofovir disoproxil with other drugs
In drug combination studies of tenofovir with nucleoside reverse transcriptase inhibitors (abacavir, didanosine, lamivudine, stavudine, zalcitabine, zidovudine), non-nucleoside reverse transcriptase inhibitors (delavirdine, efavirenz, nevirapine), and protease inhibitors (amprenavir, indinavir, nelfinavir, ritonavir, saquinavir), additive and synergistic effects were seen. Tenofovir demonstrated antiviral activities in cell cultures against HIV-1 .
Clinical Use
Nucleoside reverse transcriptase inhibitor:
Treatment of HIV in combination with other
antiretroviral drugs
Treatment of hepatitis B in compensated liver
disease
Drug interactions
Potentially hazardous interactions with other drugs
Antivirals: avoid with adefovir and cidofovir; reduces
concentration of atazanavir, also concentration of
tenofovir possibly increased; increased didanosine
concentration resulting in increased toxicity
(e.g. pancreatitis and lactic acidosis) - avoid;
concentration increased by lopinavir and telaprevir.
Co-administration with other drugs that are actively
secreted via the tubular anionic transporter.
Orlistat: absorption possibly reduced by orlistat.
Metabolism
Tenofovir disoproxil fumarate is the fumarate salt of the prodrug tenofovir disoproxil. Tenofovir disoproxil is absorbed and converted to its active form, tenofovir, a nucleoside monophosphate (nucleotide) analog. Tenofovir is then converted to the active metabolite, tenofovir diphosphate, a chain terminator, by constitutively expressed enzymes in the cell . Two phosphorylation steps are required to convert tenofovir disoproxil to the active drug form .
The cytochrome P450 enzyme system is not involved with the metabolism of tenofovir disoproxil or tenofovir .
Metabolism
Tenofovir is excreted mainly in the urine by both active tubular secretion and glomerular filtration.
Properties of Tenofovir disoproxil
| Boiling point: | 642.7±65.0 °C(Predicted) |
| Density | 1.45±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | DMSO: ≥ 38 mg/mL (73.16 mM) |
| form | Solid |
| pka | 4.20±0.10(Predicted) |
| color | White to off-white |
| CAS DataBase Reference | 201341-05-1 |
Safety information for Tenofovir disoproxil
Computed Descriptors for Tenofovir disoproxil
Tenofovir disoproxil manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![[[(1R)-2-(6-aMino-9H-purin-9-yl)-1-Methylethoxy]Methyl]-, Monophenylester](https://img.chemicalbook.in/CAS/20200611/GIF/379270-35-6.gif)
You may like
-
 Tenofovir disoproxil 99%View Details
Tenofovir disoproxil 99%View Details -
 201341-05-1 99%View Details
201341-05-1 99%View Details
201341-05-1 -
 Tenofovir disoproxil 98%View Details
Tenofovir disoproxil 98%View Details
201341-05-1 -
 Tenofovir disoproxil 201341-05-1 98%View Details
Tenofovir disoproxil 201341-05-1 98%View Details
201341-05-1 -
 Tenofovir disoproxil 98% (HPLC) CAS 201341-05-1View Details
Tenofovir disoproxil 98% (HPLC) CAS 201341-05-1View Details
201341-05-1 -
 Tenofovir disoproxil 98% CAS 201341-05-1View Details
Tenofovir disoproxil 98% CAS 201341-05-1View Details
201341-05-1 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
