Tenofovir alafenamide hemifumarate
- CAS NO.:1392275-56-7
- Empirical Formula: C25H33N6O9P
- Molecular Weight: 592.55
- MDL number: MFCD30534429
- EINECS: 805-448-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
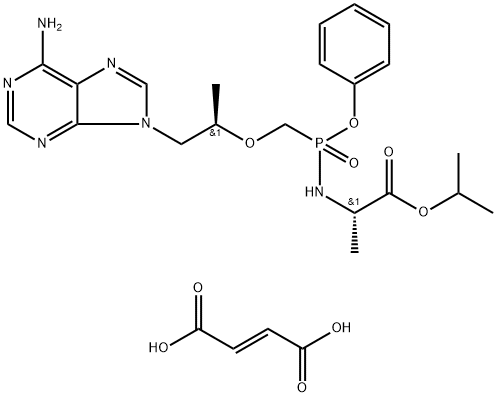
What is Tenofovir alafenamide hemifumarate?
Description
Tenofovir alafenamide hemifumarate is an investigational oral prodrug of Tenofovir. Tenofovir is a HIV-1 nucleotide reverse transcriptase inhibitor.
Chemical properties
White to almost White crystal or crystalline powder.
Originator
Gilead Sciences
The Uses of Tenofovir alafenamide hemifumarate
Tenofovir alafenamide hemifumarate is a newer, more effective prodrug used in combination with FTC that has recently been approved for the prevention of HIV transmission through the rectum. It is used to treat chronic hepatitis B virus (HBV) infection in adults with compensated liver disease.
Definition
ChEBI: Tenofovir alafenamide fumarate is a fumarate salt prepared from tenofovir alafenamide by reaction of one molecule of fumaric acid for every two molecules of tenofovir alafenamide. A prodrug for tenofovir, it is used in combination therapy for the treatment of HIV-1 infection. It has a role as an antiviral drug, a HIV-1 reverse transcriptase inhibitor and a prodrug. It contains a tenofovir alafenamide(1+).
Clinical Use
Tenofovir alafenamide hemifumarate is useful in the treatment and/or prophylaxis of one or more viral infections in man or animals, including infections caused by DNA viruses. RNA viruses, herpesviruses (e.g., CMV, HSV 1, HSV 2, VZV), retroviruses, hepadnaviruses (e.g., HBV), papillomavirus, hantavirus, adenoviruses and HIV. Like tenofovir disoproxil, tenofovir alafenamide is another prodrug form of tenofovir, and can be used in the treatment and/or prophylaxis of the same conditions.
Side Effects
Check with your doctor immediately if any of the following side effects occur:
Abdominal or stomach discomfort,bloody urine,dark urine,decreased appetite,decreased frequency or amount of urine,troubled breathing,cough,headache,back pain.
Synthesis
Under a nitrogen atmosphere, 200 g of isopropanol, 9 g of fumaric acid, and 50 g of TAF-3 were sequentially added to a 2 L reaction flask. Turn on the stirring, control the temperature at 40 ~ 50 °C, stir and dissolve, filter while hot, and collect the mother liquor. Transfer the mother liquor to a 2L reaction bottle, start stirring, control the temperature at 40 ~ 50 °C, stir and dissolve, slowly reduce the temperature to 0 ~ 5 °C, cool 2 ~ 3 hours, heat stirring for 10 hours. Filtration, drying at 60-65 °C for 12 to 16 hours, to obtain 37.5 g of Tenofovir alafenamide hemifumarate finished product, white powder, yield 75.0%.
Drug interactions
Some products that may interact with this drug are: adefovir, orlistat, other drugs that may harm the kidneys (including aminoglycosides such as amikacin/gentamicin).
Advantages
One major advantage of the hemifumarate form of tenofovir alafenamide over the monofumarate form is its exceptional capability to purge GS-7339 (i.e., 9-[(R)-2-[[(R)-[[(S)-1-(isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methoxy]propyl]adenine, which is the major diastereomeric impurity in the active pharmaceutical ingredient. Thus, the hemifumarate form of tenofovir alafenamide can be more readily and easily separated from impurities than the monofumarate form. Other major advantages of tenofovir alafenamide hemifumarate over the monofumarate form include improved thermodynamic and chemical stability (including long-term storage stability), superior process reproducibility, superior drug product content uniformity, and a higher melting point.
Properties of Tenofovir alafenamide hemifumarate
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO:75.0(Max Conc. mg/mL);70.16(Max Conc. mM) |
| form | Solid |
| color | White to off-white |
| CAS DataBase Reference | 1392275-56-7 |
Safety information for Tenofovir alafenamide hemifumarate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tenofovir alafenamide hemifumarate
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
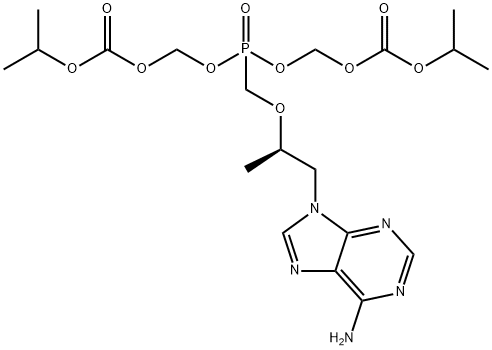
![P-[[(1S)-2-(6-AMino-9H-purin-9-yl)-1-Methylethoxy]Methyl]-phosphonic Acid Monoethyl Ester](https://img.chemicalbook.in/CAS/GIF/1255525-18-8.gif)
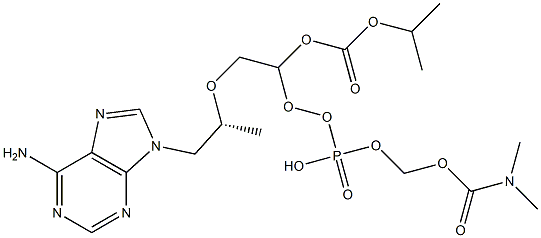
![2,4,6,8-Tetraoxa-5-phosphanonanedioic acid, 5-[[(1R)-2-[6-[[[[9-[(2R)-5-hydroxy-2,11-diMethyl-5-oxido-9-oxo-3,6,8,10-tetraoxa-5-phosphadodec-1-yl]-9H-purin-6-yl]aMino]Methyl]aMino]-9H-purin-9-yl]-1-Methylethoxy]Methyl]-, 1,9-bis(1-Methylethyl) ester, 5-ox](https://img.chemicalbook.in/CAS/GIF/1093279-77-6.gif)

You may like
-
 1392275-56-7 98%View Details
1392275-56-7 98%View Details
1392275-56-7 -
 Tenofovir Alafenamide Hemi Fumarate 97%View Details
Tenofovir Alafenamide Hemi Fumarate 97%View Details -
 Tenofovir Alafenamide Hemi fumarate 99%View Details
Tenofovir Alafenamide Hemi fumarate 99%View Details -
 1392275-56-7 Tenofovir Alafenamide fumarate 98%View Details
1392275-56-7 Tenofovir Alafenamide fumarate 98%View Details
1392275-56-7 -
 1392275-56-7 99%View Details
1392275-56-7 99%View Details
1392275-56-7 -
 Tenofovir Alafenamide fumarate 99%View Details
Tenofovir Alafenamide fumarate 99%View Details
1392275-56-7 -
 1392275-56-7 Tenofovir Alafenamide fumarate 98%View Details
1392275-56-7 Tenofovir Alafenamide fumarate 98%View Details
1392275-56-7 -
 Tenofovir Alafenamide Fumarate, Treatment: Hiv-1 InfectionView Details
Tenofovir Alafenamide Fumarate, Treatment: Hiv-1 InfectionView Details
1392275-56-7
