Tenofovir disoproxil fumarate
Synonym(s):Bis(POC)PMPA fumarate;Bis{[(Isopropoxycarbonyl)oxy]methyl} ({[(2R)-1-(6-amino-9H-purin-9-yl)-2-propanyl]oxy}methyl)phosphonate fumarate;Tenofovir disoproxil fumarate
- CAS NO.:202138-50-9
- Empirical Formula: C23H34N5O14P
- Molecular Weight: 635.52
- MDL number: MFCD08141829
- EINECS: 687-766-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-25 18:09:02
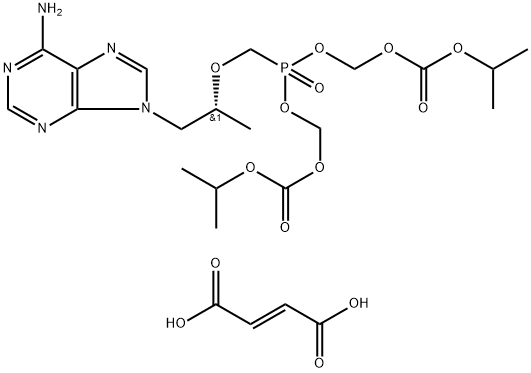
What is Tenofovir disoproxil fumarate?
Description
Tenofovir disoproxil fumarate (tenofovir DF) is the first nucleotide analog reverse transcriptase inhibitor (NRTI) to be launched in the US as a new oral treatment for HIV infection. This inhibitor can be prepared in six steps from (S)-glycidol by successive hydrogenation and intramolecular esterification to give the cyclic carbonate which reacts with adenine to afford the key hydroxypropyl adenine. The latter is transformed into the phosphonic acid [(R)-PMPA] condensed with the appropriate carbonate to give the phosphonic acid diester. Tenofovir DF is a water soluble diester that acts as a prodrug which is rapidly hydrolyzed to form the free tenofovir (analog of adenosine monophosphate) that acts only after two intracollular phosphorylation steps as a potent competitive inhibitor for reverse transcriptase. Tenofovir as the triphosphate form is then incorporated into DNA and causes DNA chain termination. In contrast to nucleoside analogs, tenofovir does not require initial intracellular phosphorylation, a limiting factor in resting cells. Tenofovir DF diffuses rapidly into cells due to its liphophilicity, unlike tenofovir, which requires an endocytosis transport process. The in vitro anti HIV activity of the oral prodrug is substantially greater than that of tenofovir alone (up to 100-fold), related to more rapid/extensive cellular uptake. Despite the fact that the prodrug is rapidly converted to free tenofovir in plasma after oral absorption, it is suggested that even small amounts of prodrug can provide enhanced antMral activity. In clinical studies, tenofovir DF has been shown to reduce the level of HIV in the blood for up to 48 weeks when added to patient's existing antiretroviral regimens. The drug also reduced viral load even in patients whose HIV had developed resistance to currently available antiretroviral drugs. Tenofovir DF is unique in demonstrating efficacy against 3TC (lamivudine)-resistant HIV strains. Moreover, the long intracellular half-life (from 12 to 50h) of the tefonovir diphosphate allowing for once daily dosing is probably an important factor in the efficacy of this drug/n vivo. The drug is eliminated by the kidney, is not metabolized by the liver and is not associated with P450 interactions. The bioavailability of the prodrug was increased significantly when taken with food from 27 to 40%. The oral bioavailabilty of tenofovir is < 10%.
Description
Tenofovir disoproxil is a prodrug of the acyclic nucleoside phosphonate, tenofovir . Tenofovir is converted by cellular enzymes to tenofovir diphosphate, an obligate chain terminator that inhibits the activity of HIV reverse transcriptase and hepatitis B virus polymerase. Due to its rapid intracellular uptake, the anti-HIV activity of tenofovir disoproxil is reportedly >100-fold greater than that of the negatively charged tenofovir in a T cell line and primary blood lymphocytes.
Chemical properties
White Solid
Originator
Gilead Sciences (US)
The Uses of Tenofovir disoproxil fumarate
Acyclic phosphonate nucleotide analogue; reverse transcriptase inhibitor. Used as an anti-HIV agent. Antiviral.
The Uses of Tenofovir disoproxil fumarate
tyrosinase inhibitor used for skin lightening and anti-melasma
The Uses of Tenofovir disoproxil fumarate
An acyclic phosphonate nucleotide analog and selective HIV-1 RT inhibitor
Definition
ChEBI: A fumarate salt prepared from equimolar amounts of tenofovir disoproxil and fumaric acid. It is used in combination therapy for the treatment of HIV infection.
Mechanism of action
Tenofovir disoproxil fumarate (TDF) is a prodrug of tenofovir. It interferes with DNA synthesis of HIV through competitive inhibition of reverse transcriptase and incorporation into viral DNA. It also inhibits hepatitis B virus polymerase, resulting in inhibition of viral replication.
brand name
Viread (Gilead Sciences).
Metabolism
Tenofovir disoproxil fumarate is rapidly converted intracellularly to tenofovir via hydrolysis, and subsequently phosphorylated to the active form, tenofovir diphosphate.
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Biochem/physiol Actions
Tenofovir disoproxil fumarate is a prodrug of tenofovir, a nucleotide analogue reverse transcriptase inhibitor (nRTI) that causes premature termination of DNA transcription. In a T cell line and primary blood lymphocytes, the antiviral activity of tenofovir disoproxil was shown to be more than 100-fold greater than tenofovir because of its rapid intracellular uptake. Tenofovir disoproxil fumarate has been used alone and in various combinations for the prevention and treatment of HIV/AIDS and chronic hepatitis B infections and is on the World Health Organization′s List of Essential Medicines.
Side Effects
Significant: Decreased bone mineral density, immune reconstitution syndrome, osteomalacia with proximal renal tubulopathy, acute renal failure and/or Fanconi syndrome.
Gastrointestinal disorders: Diarrhoea, vomiting, nausea, abdominal pain, abdominal distention, flatulence.
General disorders and administration site conditions: Asthenia, fatigue, fever.
Metabolism and nutrition disorders: Hypophosphataemia.
Nervous system disorders: Dizziness, headache.
Psychiatric disorders: Insomnia, depression.
Skin and subcutaneous tissue disorders: Rash, urticaria, pruritus.
Potentially Fatal: Lactic acidosis, severe hepatomegaly with steatosis.
References
[1] fung hb, stone ea, piacenti fj. tenofovir disoproxil fumarate: a nucleotide reverse transcriptase inhibitor for the treatment of hiv infection. clin ther. 2002 oct;24(10):1515-48.
[2] brian p. kearney, john f. flaherty, jaymin shah. tenofovir disoproxil fumarate. clinical pharmacokinetics. august 2004, volume 43, issue 9, pp 595-612
Properties of Tenofovir disoproxil fumarate
| Melting point: | 113-115°C |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | powder |
| color | white to beige |
| CAS DataBase Reference | 202138-50-9(CAS DataBase Reference) |
Safety information for Tenofovir disoproxil fumarate
Computed Descriptors for Tenofovir disoproxil fumarate
Tenofovir disoproxil fumarate manufacturer
Styrax Pharma Pvt Ltd
Lupin Ltd
Macleods Pharmaceuticals Limited
Shilpa Medicare Limited (SML)
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
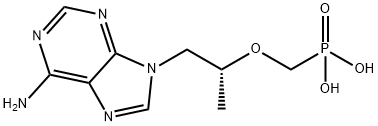
![P-[[(1S)-2-(6-AMino-9H-purin-9-yl)-1-Methylethoxy]Methyl]-phosphonic Acid Monoethyl Ester](https://img.chemicalbook.in/CAS/GIF/1255525-18-8.gif)
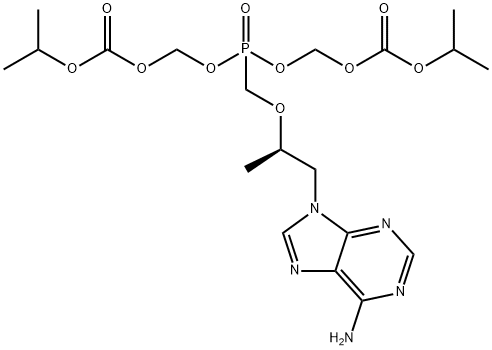
![2,4,6,8-Tetraoxa-5-phosphanonanedioic acid, 5-[[(1R)-2-[6-[[[[9-[(2R)-5-hydroxy-2,11-diMethyl-5-oxido-9-oxo-3,6,8,10-tetraoxa-5-phosphadodec-1-yl]-9H-purin-6-yl]aMino]Methyl]aMino]-9H-purin-9-yl]-1-Methylethoxy]Methyl]-, 1,9-bis(1-Methylethyl) ester, 5-ox](https://img.chemicalbook.in/CAS/GIF/1093279-77-6.gif)
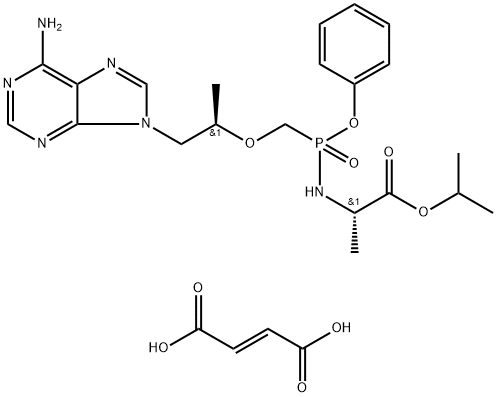
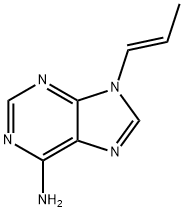

![9-[(R)-2-(Phosphonomethoxy)propyl]adenine monohydrate](https://img.chemicalbook.in/CAS/GIF/206184-49-8.gif)
You may like
-
 Tenofovir Disoproxil Fumarate 97%View Details
Tenofovir Disoproxil Fumarate 97%View Details -
 Tenofovir Disoproxil Fumarate 99%View Details
Tenofovir Disoproxil Fumarate 99%View Details -
 Tenofovir disoproxil fumarate 95% CAS 202138-50-9View Details
Tenofovir disoproxil fumarate 95% CAS 202138-50-9View Details
202138-50-9 -
 Tenofovir disoproxil fumarate 98% (HPLC) CAS 202138-50-9View Details
Tenofovir disoproxil fumarate 98% (HPLC) CAS 202138-50-9View Details
202138-50-9 -
 Tenofovir disoproxil fumarate 98%View Details
Tenofovir disoproxil fumarate 98%View Details
202138-50-9 -
 Tenofovir disoproxil fumarate 98%View Details
Tenofovir disoproxil fumarate 98%View Details -
 Tenofovir disoproxil fumarate CAS 202138-50-9View Details
Tenofovir disoproxil fumarate CAS 202138-50-9View Details
202138-50-9 -
 Tenofovir disoproxil fumarate CAS 202138-50-9View Details
Tenofovir disoproxil fumarate CAS 202138-50-9View Details
202138-50-9