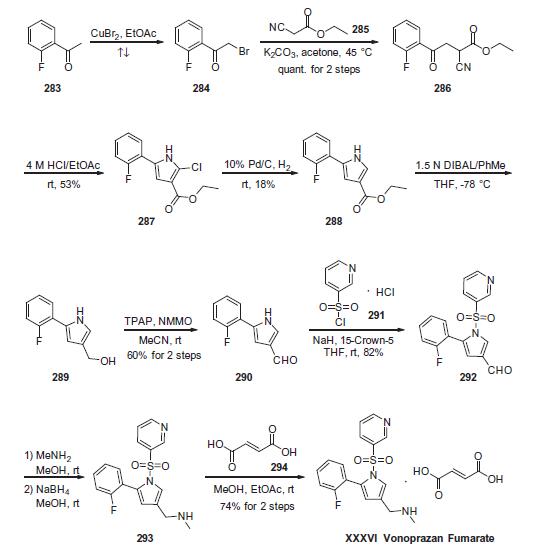
Vonoprazan fumarate
- CAS NO.:1260141-27-2
- Empirical Formula: C21H20FN3O6S
- Molecular Weight: 461.4634032
- MDL number: MFCD18633280
- EINECS: 250-635-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
What is Vonoprazan fumarate?
Description
Vonoprazan fumarate (Takecab?), discovered and developed by Takeda and Otsuka, was approved by the PMDA of Japan in December 2014, and is indicated for the treatment of gastric ulcer, duodenal ulcer and reflux esophagitis. Vonoprazan fumarate has a novel mechanism of action called potassium-competitive acid blockers, which competitively inhibit the binding of potassium ions to H+, K+-ATPase (also known as the proton pump) in the final step of gastric acid secretion in gastric parietal cells.
Bioactivity
Vonoprazan is a selective, reversible, and potassium-competitive proton pump inhibitor that inhibits gastric H+/K+ ATPase (IC50 = 17 nM) but does not inhibit porcine Na+/K+ ATPase activity when used at a concentration of 10 μM. It maintains its inhibitory effect in both weakly acidic (pH 6.5) and neutral (pH 7.5) conditions with IC50 values of 19 and 28 nM, respectively. In vivo, vonoprazan (1, 2, and 4 mg/kg) inhibits histamine-stimulated acid secretion in a dose-dependent manner in rats, with complete inhibition when administered at a dose of 4 mg/kg. It also inhibits acid secretion for more than 48 hours in dogs when administered at doses ranging from 0.1 to 1 mg/kg.
What are the applications of Application
Vonoprazan Fumarate is a novel potassium-competitive acid blocker for the treatment of acid-related diseases. It is also a proton pump inhibitor (PPI) reversibly inhibiting H+/K+, ATPase. It is mainly used in the treatment of acid-related diseases such as GERD and peptic ulcer disease.
Synthesis
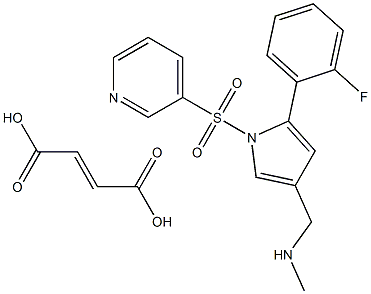
Commercially available 2-fluoroacetophenone (283) was brominated to yield a-bromo-acetophenone derivative 284. This compound was treated with ethyl 2-cyanoacetate (285) under basic conditions to provide ketoester 286 in essentially quantitative yield. Next, intramolecular condensation of 286 upon treatment of 4 M HCl furnished the tri-substituted pyrrole 287 in 53% yield. Reduction of the chloride under hydrogenolytic conditions facilitated arrival at pyrrole 288, albeit in just 18% yield. Subsequent diisobutylaluminium hydride (DIBAL) reduction, followed by the oxidation with tetrapropylammonium perruthenate (TPAP) and 4-methylmorpholine N-oxide (NMMO) afforded the corresponding aldehyde 290 in 60% yield across the 2 steps. Next, N-pyrrole substitution with pyridine- 3-sulfonyl chloride 291 gave rise to N-sulfonylpyrrole 292 in 82% yield. Reductive amination of 292 afforded amine 293, which was treated with fumaric acid (294) via co-crystallization to provide vonoprazan fumarate (XXXVI) in 74% for the two steps.
References
[1]. yasunobu hori, jun matsukawa, toshiyuki takeuchi, et al. a study comparing the antisecretory effect of tak-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. journal of pharmacology and experimental therapeutics, 2011, 337:797-804.
[2]. jun matsukawa, yasunobu hori, haruyuki nishida, et al. a comparative study on the modes of action of tak-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. biochemical pharmacology, 2011, 81:1145-1151.
Properties of Vonoprazan fumarate
| storage temp. | Store at -20°C |
| solubility | insoluble in H2O; insoluble in EtOH; ≥18.9 mg/mL in DMSO |
| form | solid |
Safety information for Vonoprazan fumarate
Computed Descriptors for Vonoprazan fumarate
| InChIKey | ROGSHYHKHPCCJW-WLHGVMLRSA-N |
| SMILES | N1(S(C2=CC=CN=C2)(=O)=O)C(C2=CC=CC=C2F)=CC(CNC)=C1.C(O)(=O)/C=C/C(O)=O |
Vonoprazan fumarate manufacturer
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 1260141-27-2 Vonoprazan Fumarate 98%View Details
1260141-27-2 Vonoprazan Fumarate 98%View Details
1260141-27-2 -
 1260141-27-2 98%View Details
1260141-27-2 98%View Details
1260141-27-2 -
 Vonoprazan Fumarate 98%View Details
Vonoprazan Fumarate 98%View Details -
 1260141-27-2 / 881681-01-2 99%View Details
1260141-27-2 / 881681-01-2 99%View Details
1260141-27-2 / 881681-01-2 -
 Vonoprazan Fumarate 98%View Details
Vonoprazan Fumarate 98%View Details
1260141-27-2 / 881681-01-2 -
 Vonoprazan Fumarate 1260141-27-2 / 881681-01-2 99%View Details
Vonoprazan Fumarate 1260141-27-2 / 881681-01-2 99%View Details
1260141-27-2 / 881681-01-2 -
 1260141-27-2 / 881681-01-2 Vonoprazan Fumarate 99%View Details
1260141-27-2 / 881681-01-2 Vonoprazan Fumarate 99%View Details
1260141-27-2 / 881681-01-2 -
 TAK-438 98% (HPLC) CAS 1260141-27-2View Details
TAK-438 98% (HPLC) CAS 1260141-27-2View Details
1260141-27-2
