Lansoprazole
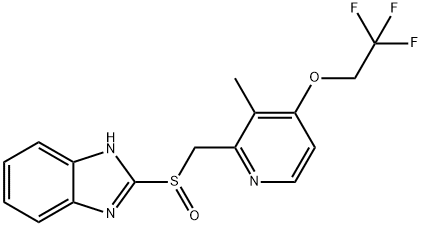
Synonym(s):2-[[[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl] methyl] sulfinyl]-1H-benzimidazole;LAN;Lansoprazole
- CAS NO.:103577-45-3
- Empirical Formula: C16H14F3N3O2S
- Molecular Weight: 369.36
- MDL number: MFCD00866873
- EINECS: 2017-001-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
What is Lansoprazole?
Absorption
The oral bioavailability of lansoprazole is reported to be 80-90% and the peak plasma concentration(Cmax) is achieved about 1.7 hours after oral dosing. Food reduces the absorption of lansoprazole (both Cmax and AUC are reduced by 50-70%); therefore, patients should be instructed to take lansoprazole before meals.
Toxicity
The most commonly reported adverse events occurring more frequently in lansoprazole treated patients compared to placebo include abdominal pain, constipation, diarrhea, and nausea. Lansoprazole is classified as Pregnancy Category B. Although there are animal studies that suggest lansoprazole does not cause harm to the fetus, there is still a paucity of human data. Hence, lansoprazole should only be administered to pregnant women if other options with more safety data have been exhausted. It is unknown if lansoprazole is excreted in human breast milk. It is worth mentioning that lansoprazole has been used safely in infants, and is therefore likely safe to use during breastfeeding.
Description
Lansoprazole is the second proton pump inhibitor approved as an antiulcer agent for the short term treatment of duodenal ulcer and gastroesophageal reflux disease. It is a proton-pump inhibitor that reduces the stomach’s ability to produce acids. It is also used to treat gastric ulcers and, along with antibiotics, to combat Helicobacter pylori infections. It is in the same drug class as omeprazole (Prilosec), another widely used heartburn remedy.
Chemical properties
White Crystalline Powder
The Uses of Lansoprazole
Lansoprazole is used as a gastric proton pump inhibitor. Treatment of gastroesophageal reflux disease (GERD), erosive esophagitis, duodenal ulcers, Helicobacter pylori (H. pylori) infections,stomach ulcers, prevention of stomach ulcers in those people taking non-steroidal anti-inflammatory drugs (NSAIDs)
Indications
Lansoprazole is used to reduce gastric acid secretion and is approved for short term treatment of active gastric ulcers, active duodenal ulcers, erosive reflux oesophagitis, symptomatic gastroesophageal reflux disease, and non-steroidal anti-inflammatory drug (NSAID) induced gastric and duodenal ulcers. It may be used in the maintenance and healing of several gastric conditions including duodenal ulcers, NSAID related gastric ulcers, and erosive esophagitis. Lansoprazole prevents recurrence of gastric ulcers in patients who have a documented history of gastric ulcers who also use NSAIDs chronically. Predictably, it is also useful in the management of hypersecretory conditions including Zollinger-Ellison syndrome. Lansoprazole is effective at eradicating H. pylori when used in conjunction with amoxicillin and clarithromycin (triple therapy) or with amoxicillin alone (dual therapy).
Definition
ChEBI: Lansoprazole is a member of benzimidazoles, a member of pyridines and a sulfoxide. It has a role as an EC 3.6.3.10 (H(+)/K(+)-exchanging ATPase) inhibitor and an anti-ulcer drug.
brand name
Prevacid (TAP);Ogast;Lanzor.
Biological Activity
Lansoprazole is a proton pump inhibitor that inhibits the H+/K+-ATPase. It inhibits K+ and H+ accumulation in gastric microsomes in a concentration-dependent manner (IC50s = 6.3 and 7.0 μM, respectively) and inhibits H+/K+-ATPase activity by approximately 60% when used at a concentration of 10 μM. Lansoprazole inhibits the H+/K+-ATPase in parietal cells, thus inhibiting gastric acid secretion and increasing intragastric pH. It is a substituted benzimidazole that binds covalently to proton pumps, providing complete and prolonged inhibition of acid secretion.
Pharmacokinetics
Lansoprazole decreases gastric acid secretion by targeting H+,K+-ATPase, which is the enzyme that catalyzes the final step in the acid secretion pathway in parietal cells. Conveniently, lansoprazole administered any time of day is able to inhibit both daytime and nocturnal acid secretion. The result is that lansoprazole is effective at healing duodenal ulcers, reduces ulcer-related pain, and offers relief from symptoms of heartburn Lansoprazole also reduces pepsin secretion, making it a useful treatment option for hypersecretory conditions such as Zollinger-Ellison syndrome.
Metabolism
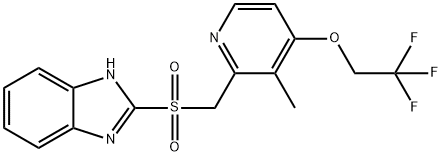
Lansoprazole is predominantly metabolized in the liver by CYP3A4 and CYP2C19. The resulting major metabolites are 5-hydroxy lansoprazole and the sulfone derivative of lansoprazole.
Properties of Lansoprazole
| Melting point: | 178-182°C dec. |
| Boiling point: | 555.8±60.0 °C(Predicted) |
| Density | 1.50±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, soluble in anhydrous ethanol, very slightly soluble in acetonitrile. |
| form | powder |
| pka | pKa 2.34±0.37 (Occasionally);3.53±0.37 (Occasionally);8.48±0.30 (Occasionally) |
| color | off-white |
| Merck | 14,5362 |
| CAS DataBase Reference | 103577-45-3(CAS DataBase Reference) |
Safety information for Lansoprazole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lansoprazole
| InChIKey | MJIHNNLFOKEZEW-UHFFFAOYSA-N |
Lansoprazole manufacturer
Gangwal Healthcare Pvt Ltd
Clickchem Research LLP
SRINI PHARMACEUTICALS PVT LTD
Yashica Pharmaceuticals Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![2-[(4-TRIFLUOROETHOXY-3,5-DIMETHYL-2-PYRIDINYL)-METHYLSULFINYL]-BENZIMIDAZOLE](https://img.chemicalbook.in/CAS/GIF/103577-47-5.gif)

![LANSOPRAZOLE RELATED COMPOUND A2-[[[3-METHYL-4-(2,2,2-TRIFLOUROETHOXY)-2-PYRIDYL]METHYL]SULFONYL]BENZIMIDAZOLE USP(CRM STANDARD)](https://img.chemicalbook.in/)

You may like
-
 Lansoprazole 98%View Details
Lansoprazole 98%View Details -
 Lansoprazole 99%View Details
Lansoprazole 99%View Details -
 Lansoprazole 98%View Details
Lansoprazole 98%View Details -
 Lansoprazole 98%View Details
Lansoprazole 98%View Details -
 Lansoprazole 98%View Details
Lansoprazole 98%View Details -
 Lansoprazole 98%View Details
Lansoprazole 98%View Details -
 Lansoprazole, Packaging Type: Alu-Alu, For HospitalView Details
Lansoprazole, Packaging Type: Alu-Alu, For HospitalView Details
103577-45-3 -
 Lansoprazole ApiView Details
Lansoprazole ApiView Details
103577-45-3
