Vonoprazan FuMarate
- CAS NO.:881681-01-2
- Empirical Formula: C21H20FN3O6S
- Molecular Weight: 461.46
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
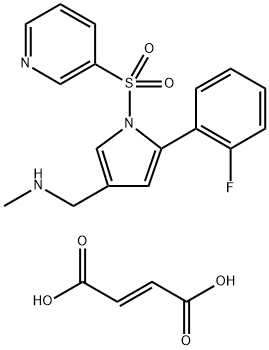
What is Vonoprazan FuMarate?
Chemical properties
Vonoprazan fumarate is white to nearly white crystals or crystalline powder which melts at 194.8°C . It can be dissolved in dimethyl sulfoxide, but has limited solubility in N,N-dimethylacetamide, N,N-dimethylformamide, methanol, and water. It is only slightly soluble in ethanol (99.5%), and has very low solubility in 2-propanol, acetone, 1-octanol, and acetonitrile.
The Uses of Vonoprazan FuMarate
Vonoprazan Fumarate (CAS# 881681-01-2) is a useful research chemical and a ATPase potassium-competitive acid blocker.
Benefits
Compared with traditional irreversible proton pump inhibitors (such as omeprazole, esomeprazole, etc.), Vonoprazan fumarate advantages:
① The onset of action is good, and the maximum acid-suppressing effect will be achieved on the first day of administration;
② Oral administration, not affected by gastric acid damage;
③It can improve the phenomenon of acid breakthrough at night.
General Description
Vonoprazan fumarate (TAK-438) is a new oral anti-gastric acid drug jointly launched by Takeda Pharmaceutical and Otsuka Pharmaceutical. It can be used for the treatment of erosive esophagitis, gastric ulcer and duodenal ulcer.
Mechanism of action
Vonoprazan fumarate is a potassium ion (K+) competitive acid blocker (P-CAB), which is a reversible proton pump inhibitor. It can inhibit the combination of K+ and H+-K+-ATPase (proton pump), so that secretion of gastric acid is terminated. Vonoprazan fumarate has a strong and lasting inhibitory effect on gastric acid secretion.
Synthesis
Vonoprazan FuMarate is prepared by the reaction of vonoprazan and (2E)-but-2-enedioic acid. The steps are as follows:
Step 1: (2E)-but-2-enedioic acid With sodium hydroxide In water at 50 - 60℃.
Step 2: vonoprazan In water; toluene at 25 - 35℃; Reagent/catalyst.
Add 730g of water and 73g of fumaric acid to a 2L reaction flask at 5060, stir and disperse, control the temperature between 5060, add 25g of hydrogen hydroxide dropwise to the fumaric acid suspension The solution of sodium and 300 g of water was gradually dissolved in the system during the dropwise addition, and the aqueous solution of compound was obtained and kept for use.Between 25 and 35°C, take half of the toluene solution of compound obtained in the previous step and put it into a 5L reaction flask, and add the aqueous solution of the above compound dropwise to it. During the dropwise addition, the system has a white solid precipitation. After the dropwise addition was completed, keep stirring for 2-4 hours, filter, and dry the filter cake at 50°C to obtain 131.32 g of white solid, the HPLC purity was 99.96%, and the yield was 94%.
Properties of Vonoprazan FuMarate
| storage temp. | Store at -20°C |
| solubility | DMSO: 50 mg/mL (108.35 mM) |
| form | Solid |
| color | White to Off-White |
Safety information for Vonoprazan FuMarate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Vonoprazan FuMarate
| InChIKey | ROGSHYHKHPCCJW-WLHGVMLRSA-N |
| SMILES | C(/C(=O)O)=C\C(=O)O.S(C1=CN=CC=C1)(N1C=C(CNC)C=C1C1C=CC=CC=1F)(=O)=O |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 881681-01-2 Vonoprazan Fumarate In-House 98%View Details
881681-01-2 Vonoprazan Fumarate In-House 98%View Details
881681-01-2 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1