Pantoprazole
- CAS NO.:102625-70-7
- Empirical Formula: C16H15F2N3O4S
- Molecular Weight: 383.37
- MDL number: MFCD00870182
- EINECS: 600-331-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-06 22:41:32
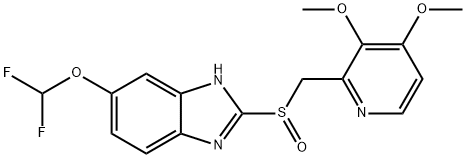
What is Pantoprazole?
Absorption
Pantoprazole is absorbed after oral administration as an enteric-coated tablet with maximum plasma concentrations attained within 2 – 3 hours and a bioavailability of 77% that does not change with multiple dosing . Following an oral dose of 40mg, the Cmax is approximately 2.5 μg/mL with a tmax of 2 to 3 hours. The AUC is approximately 5 μg.h/mL. There is no food effect on AUC (bioavailability) and Cmax.
Delayed-release tablets are prepared as enteric-coated tablets so that absorption of pantoprazole begins only after the tablet leaves the stomach.
Toxicity
Rat Oral LD 50 747 mg/kg
Tumorigenicity
Because of the chronic nature of GERD, there may be a potential for long-term administration of pantoprazole. In long-term rodent studies, pantoprazole was carcinogenic and its administration lead to rare types of gastrointestinal tumors. The relevance of these findings to tumor development in humans is unknown at this time.
Teratogenic Effects
This drug falls under pregnancy category B category. Reproduction studies have been performed in rats at oral doses up to 88 times the recommended human dose (RHD), as well as in rabbits at oral doses up to 16 times the RHD, and have shown no evidence of impaired fertility or harm to the fetus caused by pantoprazole. No adequate and well-controlled studies in pregnant women have been completed. Because animal reproduction studies are not always predictive of human response, this drug should only be used during pregnancy if clearly required.
Nursing Mothers
Pantoprazole and its metabolites have been found to be excreted in the milk of rats. Pantoprazole excretion in human milk has been found in a study performed with a single nursing mother after one 40 mg oral dose. The clinical relevance of this finding is not known, however, it is advisable to take note of this finding when considering pantoprazole use during nursing. Many drugs excreted in human breastmilk have a risk for serious adverse effects in nursing infants.
Description
Pantoprazole, an irreversible proton pump inhibitor, reached its first market worldwide in Germany for acute treatment of gastric and duodenal ulcers and gastroesophageal reflux disease. Proton pump inhibitors are more effective than other strategies in inhibiting acid secretion since they function at the final step of acid production, therefore, provide superior symptom relief and healing in all acid related diseases. As the third substituted benzimidazole proton pump inhibitor marketed, pantoprazole not only has superior ulcer-preventing effect but also is more potent than omeprazole in healing acetic acid-induced gastric and duodenal ulcers with extremely low acute toxicity. The mechanism of action for this class of compounds has been suggested to be mediated via the protonated form of the molecule which selectively reacts with cysteines present on the extracytoplasmic face of the enzyme to form covalent disulfide bonds.
Originator
Byk Gulden (Germany)
The Uses of Pantoprazole
proton pump inhibitor, gastric acid release inhibitor, antiulcer
Background
Pantoprazole is a first-generation proton pump inhibitor (PPI) used for the management of gastroesophageal reflux disease (GERD), for gastric protection to prevent recurrence of stomach ulcers or gastric damage from chronic use of NSAIDs, and for the treatment of pathological hypersecretory conditions including Zollinger-Ellison (ZE) Syndrome. It can also be found in quadruple regimens for the treatment of H. pylori infections along with other antibiotics including amoxicillin, clarithromycin, and metronidazole, for example. Its efficacy is considered similar to other medications within the PPI class including omeprazole, esomeprazole, lansoprazole, dexlansoprazole, and rabeprazole.
Pantoprazole exerts its stomach acid-suppressing effects by preventing the final step in gastric acid production by covalently binding to sulfhydryl groups of cysteines found on the (H+, K+)-ATPase enzyme at the secretory surface of gastric parietal cell. This effect leads to inhibition of both basal and stimulated gastric acid secretion, irrespective of the stimulus. As the binding of pantoprazole to the (H+, K+)-ATPase enzyme is irreversible and new enzyme needs to be expressed in order to resume acid secretion, pantoprazole's duration of antisecretory effect persists longer than 24 hours.
Due to their good safety profile and as several PPIs are available over the counter without a prescription, their current use in North America is widespread. Long term use of PPIs such as pantoprazole have been associated with possible adverse effects, however, including increased susceptibility to bacterial infections (including gastrointestinal C. difficile), reduced absorption of micronutrients including iron and B12, and an increased risk of developing hypomagnesemia and hypocalcemia which may contribute to osteoporosis and bone fractures later in life.
PPIs such as pantoprazole have also been shown to inhibit the activity of dimethylarginine dimethylaminohydrolase (DDAH), an enzyme necessary for cardiovascular health. DDAH inhibition causes a consequent accumulation of the nitric oxide synthase inhibitor asymmetric dimethylarginie (ADMA), which is thought to cause the association of PPIs with increased risk of cardiovascular events in patients with unstable coronary syndromes.
Pantoprazole doses should be slowly lowered, or tapered, before discontinuing as rapid discontinuation of PPIs such as pantoprazole may cause a rebound effect and a short term increase in hypersecretion.
Indications
Pantoprazole Injection:
Treatment of gastroesophageal reflux disease associated with a history of erosive esophagitis
Pantoprazole for injection is indicated for short-term treatment (7-10 days) of patients having gastroesophageal reflux disease (GERD) with a history of erosive esophagitis, as an alternative to oral medication in patients who are unable to continue taking pantoprazole delayed-release tablets. Safety and efficacy of pantoprazole injection as the initial treatment of patients having GERD with a history of erosive esophagitis have not been demonstrated at this time.
Pathological Hypersecretion Associated with Zollinger-Ellison Syndrome
Pantoprazole for injection is indicated for the treatment of pathological hypersecretory conditions associated with Zollinger-Ellison Syndrome or other neoplastic conditions.
Pantoprazole delayed-release oral suspension:
Short-Term Treatment of erosive esophagitis associated with gastroesophageal reflux disease (GERD)
Indicated in adults and pediatric patients five years of age and above for the short-term treatment (up to 8 weeks) in the healing and symptomatic relief of erosive esophagitis. For adult patients who have not healed after 8 weeks of treatment, an additional 8-week course of pantoprazole may be considered. Safety of treatment beyond 8 weeks in pediatric patients has not been determined.
Maintenance of healing of erosive esophagitis
Indicated for maintenance of healing of erosive esophagitis and reduction in relapse rates of daytime and nighttime heartburn symptoms in adult patients with GERD.
Pathological hypersecretory conditions including Zollinger-Ellison syndrome
Indicated for the long-term treatment of the above conditions.
What are the applications of Application
Pantoprazole is an inhibitor of gastric H+/K+-ATPase
Definition
ChEBI: A member of the class of benzimidazoles that is 1H-benzimidazole substituted by a difluoromethoxy group at position 5 and a [(3,4-dimethoxypyridin-2-yl)methyl]sulfinyl group at position 2.
Manufacturing Process
2-Chloromethyl-4,5-dimethoxy-3-methylpyridinium chloride (about 1.5 g) are added to a solution of 5-difluoromethoxy-1H-benzimidazole-2-thiol in 10 ml of ethanol and 10 ml of 1 N sodium hydroxide solution. The yellow reaction mixture is stirred at 20°C for 1 hour, a further 10 ml of water are added,
whereupon a colorless solid precipitates out, the mixture is stirred for a further 5 hours and filtered and the residue is rinsed with 1 N sodium hydroxide solution and water and dried to constant weight. The 5difluoromethoxy-2-[(4,5-dimethoxy-2-pyridyl)methylthio]-1H-benzimidazole is obtained as an oil.
5-Difluoromethoxy-2-[(4,5-dimethoxy-2-pyridyl)methylthio]-1H-benzimidazole (about 1 g) are dissolved in 10 ml of dioxane and 2 ml of 1 N sodium hydroxide solution. An equimolar amount of a titrated aqueous sodium hypochlorite solution, to which 1 mole per liter of sodium hydroxide solution has been added, is first added dropwise, while cooling with ice. After one hour a further equivalent and after 3 hours half the equimolar amount of sodium hypochlorite are added, to achieve complete reaction. After a reaction time of 4 hours, 5 ml of 5% strength sodium thiosulfate solution and another 25 ml of dioxane are added and the upper dioxane phase is separated off, washed once with 5 ml of sodium thiosulfate solution and concentrated on a rotary evaporator. The oily residue is dissolved in 20 ml of water and 10 ml of ethyl acetate and the solution is brought to pH 7 with about 100 ml of a buffer solution of pH 6.8. The solid which has precipitated out is filtered off with suction over a suction filter, washed with water, extracted by stirring at 0C with acetone and dried. 5-Difluoromethoxy-2-[(4,5-dimethoxy-2pyridyl)methanesulfinyl]-1H-benzimidazole is prepared; yield about 85%.
brand name
Pantozol; Rifun
Therapeutic Function
Antiulcer
Pharmacokinetics
This drug acts to decrease gastric acid secretion, which reduces stomach acidity. Pantoprazole administration leads to long-lasting inhibition of gastric acid secretion.
General Effects
Pantoprazole has been shown to reduce acid reflux-related symptoms, heal inflammation of the esophagus, and improve patient quality of life more effectively than histamine-2 receptor antagonists (H2 blockers). This drug has an excellent safety profile and a low incidence of drug interactions. It can be used safely in various high-risk patient populations, including the elderly and those with renal failure or moderate hepatic dysfunction.
Due to their good safety profile and as several PPIs are available over the counter without a prescription, their current use in North America is widespread. Long term use of PPIs such as pantoprazole have been associated with possible adverse effects, however, including increased susceptibility to bacterial infections (including gastrointestinal C. difficile), reduced absorption of micronutrients including iron and B12, and an increased risk of developing hypomagnesemia and hypocalcemia which may contribute to osteoporosis and bone fractures later in life.
PPIs such as pantoprazole have also been shown to inhibit the activity of dimethylarginine dimethylaminohydrolase (DDAH), an enzyme necessary for cardiovascular health. DDAH inhibition causes a consequent accumulation of the nitric oxide synthase inhibitor asymmetric dimethylarginie (ADMA), which is thought to cause the association of PPIs with increased risk of cardiovascular events in patients with unstable coronary syndromes .
A note on laboratory testing abnormalities
During treatment with antisecretory medicinal products such as pantoprazole, serum gastrin (a peptide hormone that stimulates secretion of gastric acid) increases in response to the decreased acid secretion caused by proton pump inhibition. The increased gastrin level may interfere with investigations for neuroendocrine tumors. Published evidence suggests that proton pump inhibitors should be stopped 14 days before chromogranin A (CgA) measurements. This permits chromogranin A levels, that might be falsely elevated after proton pump inhibitor treatment, to return to the normal reference range.
Reports have been made of false-positive results in urine screening tests for tetrahydrocannabinol (THC) in patients receiving the majority of proton pump inhibitors, including pantoprazole. A confirmatory method should be used.
Clinical Use
Pantoprazole
Veterinary Drugs and Treatments
Pantoprazole may be useful in treating or preventing gastric acidrelated
pathologies in dogs, cats, foals and camelids, particularly
when the intravenous route is preferred. Pantoprazole is available
in both intravenous and oral tablet (delayed-release) formulations.
One study (Bersenas, Mathews et al. 2005) performed in dogs, comparing
the gastric pH effects of intravenous pantoprazole with oral
omeprazole, intravenous ranitidine, and intravenous famotidine,
found at the dosages used, that pantoprazole was more effective
than ranitidine, but similar to famotidine, and that oral omeprazole
was more effective in maintaining intragastric pH >3 for a longer
period than pantoprazole.
Pantoprazole has been shown to directly reduce in vitro counts
of H. pylori and is used in some H. pylori treatment protocols for
humans.
Drug interactions
Potentially hazardous interactions with other drugs
Anticoagulants: effect of coumarins possibly
enhanced.
Antifungals: absorption of itraconazole and
ketoconazole reduced; avoid with posaconazole.
Antivirals: concentration of atazanavir and rilpivirine
reduced - avoid; concentration of raltegravir and
saquinavir possibly increased - avoid.
Clopidogrel: possibly reduced antiplatelet effect.
Cytotoxics: possibly reduced excretion of
methotrexate; avoid with dasatinib, erlotinib and
vandetanib; possibly reduced lapatinib absorption;
possibly reduced absorption of pazopanib.
Ulipristal: reduced contraceptive effect, avoid with
high dose ulipristal.
Metabolism
Pantoprazole is heavily metabolized in the liver by the cytochrome P450 (CYP) system. Pantoprazole metabolism is independent of the route of administration (intravenous or oral). The main metabolic pathway is demethylation, by CYP2C19 hepatic cytochrome enzyme, followed by sulfation; other metabolic pathways include oxidation by CYP3A4. There is no evidence that any of the pantoprazole metabolites are pharmacologically active.
After hepatic metabolism, almost 80% of an oral or intravenous dose is excreted as metabolites in urine; the remainder is found in feces and originates from biliary secretion.
Metabolism
Pantoprazole is extensively metabolised in the liver,
primarily by the cytochrome P450 isoenzyme CYP2C19,
to desmethylpantoprazole; small amounts are also
metabolised by CYP3A4, CYP2D6, and CYP2C9.
Metabolites are excreted mainly in the urine, with the
remainder being excreted in faeces via the bile.
References
[1] jungnickel p w. pantoprazole: a new proton pump inhibitor. clinical therapeutics, 2000, 22(11): 1268-1293.
[2] sachs g, shin j m, howden c w. review article: the clinical pharmacology of proton pump inhibitors. alimentary pharmacology & therapeutics, 2006, 23(s2): 2-8.
Properties of Pantoprazole
| Melting point: | 139-140° (dec) |
| Boiling point: | 586.9±60.0 °C(Predicted) |
| Density | 1.51±0.1 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Chloroform (Slightly), Methanol (Slightly, Heated, Sonicated) |
| form | Solid |
| pka | pKa1 3.92; pKa2 8.19(at 25℃) |
| color | Off-White to Pale Orange |
| InChI | InChI=1S/C16H15F2N3O4S/c1-23-13-5-6-19-12(14(13)24-2)8-26(22)16-20-10-4-3-9(25-15(17)18)7-11(10)21-16/h3-7,15H,8H2,1-2H3,(H,20,21) |
| CAS DataBase Reference | 102625-70-7(CAS DataBase Reference) |
Safety information for Pantoprazole
Computed Descriptors for Pantoprazole
| InChIKey | IQPSEEYGBUAQFF-UHFFFAOYSA-N |
| SMILES | C1(S(CC2=NC=CC(OC)=C2OC)=O)NC2=CC(OC(F)F)=CC=C2N=1 |
Pantoprazole manufacturer
Prachi Pharmaceuticals Pvt Ltd
Sainor Life Sciences Pvt Ltd
Ralington Pharma
PROTECH TELELINKS
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran



You may like
-
 102625-70-7 98%View Details
102625-70-7 98%View Details
102625-70-7 -
 Pantoprazole 98%View Details
Pantoprazole 98%View Details -
 Pantoprazole 98%View Details
Pantoprazole 98%View Details
102625-70-7 -
 Pantoprazole 98%View Details
Pantoprazole 98%View Details
102625-70-7 -
 Pantoprazole 98%View Details
Pantoprazole 98%View Details -
 Pantoprazole 99%View Details
Pantoprazole 99%View Details -
 Pantoprazole 99%View Details
Pantoprazole 99%View Details
102625-70-7 -
 102625-70-7 Pantoprazole EC Pellets 98%View Details
102625-70-7 Pantoprazole EC Pellets 98%View Details
102625-70-7