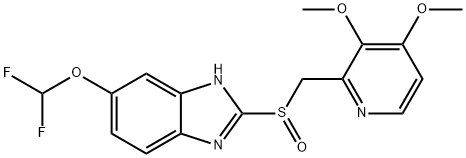
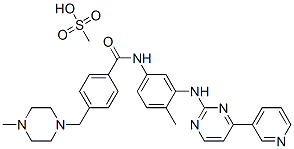
R-(+)-Lansoprazole
Synonym(s):(R)-(+)2-([3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl)-1H-benzo[d]imidazole;(R)-(+)-Lansoprazole
- CAS NO.:138530-94-6
- Empirical Formula: C16H14F3N3O2S
- Molecular Weight: 369.36
- MDL number: MFCD13196699
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:11:56

What is R-(+)-Lansoprazole?
Absorption
After oral administration, the peak plasma concentration increases approximately dose proportionally. The dual delayed release formulation achieves two plasma concentration peaks, where the first peak occurs one to two hours after administration, followed by a second peak within four to five hours. The delivery technology of dexlansoprazole MR is designed to release the drug in two separate pH-dependent phases, the first in the proximal duodenum (25% of total drug dose) and the second (75% of total drug dose) in the more distal small intestine. The median time (Tmax) to peak plasma concentrations (Cmax) of 30 mg dexlansoprazole was 4 hours and ranged from 1 to 6 hours with the Cmax value of 688 ng/mL. AUC was found to be 3275 (ng?h/mL).
Toxicity
Oral LD50 value in mice, rats and dogs is > 5,000 mg/kg. Most commonly reported adverse reactions are diarrhea, abdominal pain, nausea, upper respiratory tract infection, vomiting, and flatulence. There are no reports of significant overdose but serious adverse events of hypertension have been reported in association with twice daily doses of DEXILANT 60 mg. Nonclicnial toxicology of dexlansopraole was assessed using lansoprazole studies. In two 24-month carcinogenicity studies involving rats, lansoprazole induced dose-related gastric ECL cell hyperplasia and ECL cell carcinoids and increased the incidence of intestinal metaplasia of the gastric epithelium in both sexes of rats. Dexlansoprazole is expected to have no effect on fertility and the reproductive system.
Description
The mechanism of PPIs involves the irreversible binding to the hydrogen/potassium adenosine triphosphatase enzyme system, commonly referred to as the gastric proton pump, of the gastric parietal cell. As the last stage in gastric acid secretion, blockade of the gastric proton pump is an effective treatment for a variety of diseases requiring acid suppression, such as heartburn, peptic ulcers, and GERD. Dexlansoprazole is the latest PPI to hit the market, joining the ranks of omeprazole, rabeprazole, pantoprazole, esomeprazole, and lansoprazole, and is the Renantiomer of the racemic lansoprazole. Compared to its predecessors, dexlansoprazole exhibits improved pharmacokinetics with slower clearance and longer terminal half-life. In addition, dexlansoprazole utilizes a novel DDR technology; drug release is optimized through the use of granules with different pH-dependent dissolution profiles, thereby providing an initial release in the proximal small intestine within 1-2 h of administration followed by a subsequent release at distal regions of the small intestine several hours later. With its longer duration of action culminating in more effective acid suppression, dexlansoprazole may have an advantage over conventional PPIs that possess single release formulations (immediate or delayed). Similar to all PPIs, dexlansoprazole is a prodrug that consists of pyridine and benzimidazole rings with a latent sulfenamide moiety. In order to form the disulfide bond with cysteine residues of the proton pump, dexlansoprazole must be activated through two protonations followed by a spontaneous rearrangement to unmask the sulfenamide.
Description
Lansoprazole is a proton pump inhibitor that irreversibly inactivates the H+/K+-stimulated ATPase pumps in parietal cells, inhibiting gastric acid secretion and increasing intragastric pH. It is a 1:1 racemic mixture of (R)-lansoprazole and (S)-lansoprazole, both of which are pharmacologically active. (R)-Lansoprazole is an enantiomerically pure form of lansoprazole. It can inhibit acid formation in isolated canine parietal cells with an IC50 value of 59 nM and inhibit the H+/K+-ATPase with an IC50 value of 4.2 μM.
Chemical properties
Brown Solid
Originator
Takeda (Japan)
The Uses of R-(+)-Lansoprazole
The R-enantiomer of Lansoprazole; a gastric proton pump inhibitor. An antiulcerative
The Uses of R-(+)-Lansoprazole
antiulcer, proton pump inhibitor
The Uses of R-(+)-Lansoprazole
Acts as a gastric proton pump inhibitor and an antiulcerative
Background
Dexlansoprazole is a new generation proton pump inhibitor (PPI) used for the management of symptoms associated with gastroesophageal reflux disease (GERD) and erosive esophagitis. Dexlansoprazole is the R-enantiomer of Lansoprazole, which is composed of a racemic mixture of the R- and S-enantiomers. Compared to the older generation of PPIs (which includes Pantoprazole, Omeprazole, and Lansoprazole) , dexlansoprazole MR has a unique pharmacokinetic profile due to its delayed-release and dual-delivery release system. The active ingredient is released in two phases at different pH values and at different time points, resulting in two peak concentrations in the blood; 25% of the dose is released at pH 5.5 in the proximal duodenum, while the remaining 75% is released at pH 6.75 in the distal small intestine . As a result, dexlansoprazole has a peak concentration within 1-2 hours after dosing and another within 4-5 hours . Dexlansoprazole's unique pharmacokinetics addresses limitations of the older generation PPIs including short plasma half-life, break-through symptoms, and need for meal-associated dosing . These characteristics make dexlansoprazole a good option for people who struggle with adherence and strict dosage timing before meals.
Dexlansoprazole exerts its stomach acid-suppressing effects in the same way as other drugs in the PPI family by inhibiting the final step in gastric acid production. Dexlansoprazole targets the (H+, K+)-ATPase enzyme, which is involved in the secretion of hydrochloric acid through the exchange of H+ ions from the cytoplasm for K+ ions. Normally functioning (H+, K+)-ATPase stimulates hydrochloric acid secretion into the gastric lumen thereby increasing stomach acidity and lowering pH. Once absorbed into circulation, dexlansoprazole covalently binds to the sulfhydryl groups of cysteines found on the (H+, K+)-ATPase enzyme at the secretory surface of gastric parietal cells, which leads to inhibition of both basal and stimulated gastric acid secretion. Despite dexlansoprazole's unique pharmacokinetic profile, efficacy in management of GERD symptoms is considered similar to other medications within the PPI class including Omeprazole, Esomeprazole, Lansoprazole, Pantoprazole, and Rabeprazole.
Due to their good safety profile and as several PPIs are available over the counter without a prescription, their current use in North America is widespread. Long term use of PPIs such as dexlansoprazole have been associated with possible adverse effects, however, including increased susceptibility to bacterial infections (including gastrointestinal C. difficile), reduced absorption of micronutrients including iron and B12, and an increased risk of developing hypomagnesemia and hypocalcemia which may contribute to osteoporosis and bone fractures later in life . PPIs such as dexlansoprazole have also been shown to inhibit the activity of dimethylarginine dimethylaminohydrolase (DDAH), an enzyme necessary for cardiovascular health. DDAH inhibition causes a consequent accumulation of the nitric oxide synthase inhibitor asymmetric dimethylarginine (ADMA), which is thought to cause the association of PPIs with increased risk of cardiovascular events in patients with unstable coronary syndromes .
Dexlansoprazole doses should be slowly lowered, or tapered, before discontinuing as rapid discontinuation of PPIs such as dexlansoprazole may cause a rebound effect and a short term increase in hypersecretion .
Indications
Dexlansoprazole is a proton pump inhibitor (PPI) indicated in patients 12 years of age and older for:
What are the applications of Application
(R)-Lansoprazole is acts as a gastric proton pump inhibitor and an antiulcerative
Definition
ChEBI: Dexlansoprazole is a sulfoxide and a member of benzimidazoles.
brand name
Kapidex
Pharmacokinetics
Dexlansoprazole is a proton pump inhibitor (PPI) and is included in the drug class of antisecretory compounds. It blocks the final step of gastric acid secretion by specific inhibition of the (H+, K+)-ATPase at the secretory surface of the parietal cells on gastric mucosa.
Clinical Use
Takeda Pharmaceuticals received approval of dexlansoprazole, a dual release formulation of the (R)-isomer of lansoprazol proton pump inhibitor (PPI) already in the market, from the FDA in January 2009. Dexlansoprazole is a delayed release capsule for the oncedaily, oral treatment of heartburn associated with symptomatic non-erosive gastroesophageal reflux disease (GERD), the healing of erosive esophagitis (EE) and the maintenance of healed EE. The dual release formulation is designed to provide two separate releases of medication, one at 1–2 h and then another at 4–5 h after treatment, for extended efficacy in the treatment of GERD.
Side Effects
The most commonly recorded adverse reactions that occurred at a higher incidence than placebo were diarrhea, abdominal pain, nausea, vomiting, flatulence, and upper respiratory tract infection. As dexlansoprazole inhibits gastric acid secretion, its use is expected to interfere with the absorption of drugs with pH-dependent oral bioavailability. Since the HIV protease inhibitor atazanavir is dependent on gastric acid for absorption, dexlansoprazole should not be co-administered with atazanavir to avoid a loss of therapeutic efficacy. While co-administration of dexlansoprazole did not affect the pharmacokinetics of warfarin or INR (international normalized ratio: the ratio of a patient s prothrombin time to a normal sample), there have been reports of increased INR and prothrombin time in patients receiving concomitant treatment with PPIs and warfarin. Since increases in INR and prothrombin time may lead to abnormal bleeding and possibly death, concomitant use of dexlansoprazole and warfarin may necessitate monitoring for increases in INR and prothrombin time.
Synthesis
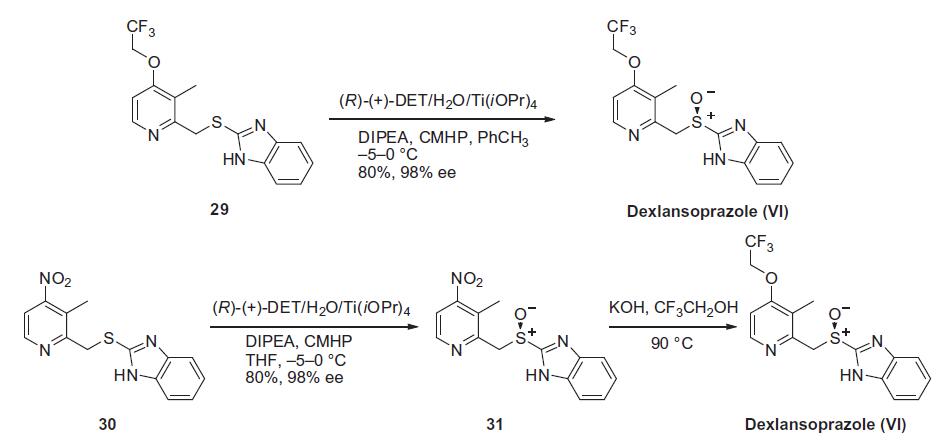
Similar to the synthesis of the chiral sulfoxide of armodafinil vide supra, the preparation of the chiral sulfoxide of lansoprazole utilized the catalytic oxidation method developed by Kagan and co-workers (the Scheme). Two routes have been reported that describe the preparation of dexlansoprazole on large scale. The first route developed by Takeda reacts commercially available thioether 29, also used to make lansoprazole, under the Kagan asymmetric oxidation conditions and the alternative route utilizes the cheaper commercial intermediate nitrosulfide 30 in the analogous asymmetric oxidation by Kagan). Thus, the catalyst complex consisting of (+)-DET, Ti(OiPr)4 and water was formed in the presence of thioether 29 in toluene at 30¨C40??C. The reaction mixture was then cooled to 5 ??C and DIPEA and cumene hydroperoxide (CMHP) were added to give, after aqueous work-up and in situ crystallization from the organic layer, dexlansoprazole (VI) in 98% ee. No yield was given in the patent. An alternate, but similar, sequence was also described wherein the nitrosulfide intermediate 30 was subjected to similar oxidative conditions that gave intermediate nitro compound 31 in 80% yield and 98% ee. Compound 31 was treated with KOH and trifluoroethanol to provide dexlansoprazole (VI).
Metabolism
Dexlansoprazole is extensively metabolized in the liver by oxidation, reduction, and subsequent formation of sulfate, glucuronide and glutathione conjugates to inactive metabolites. Oxidative metabolites are formed by the cytochrome P450 (CYP) enzyme system including hydroxylation mainly by CYP2C19, and oxidation to the sulfone by CYP3A4. Dexlansoprazole is the major circulating component in plasma regardless of CYP2C19 metabolizer status. In CYP2C19 intermediate and extensive metabolizers, the major plasma metabolites are 5-hydroxy dexlansoprazole and its glucuronide conjugate, while in CYP2C19 poor metabolizers dexlansoprazole sulfone is the major plasma metabolite.
Properties of R-(+)-Lansoprazole
| Melting point: | 66-68?C |
| Boiling point: | 555.8±60.0 °C(Predicted) |
| Density | 1.50±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.56±0.10(Predicted) |
| color | Off-White to Dark Brown |
| Stability: | Hygroscopic |
Safety information for R-(+)-Lansoprazole
Computed Descriptors for R-(+)-Lansoprazole
R-(+)-Lansoprazole manufacturer
SRINI PHARMACEUTICALS PVT LTD
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Dexlansoprazole 99%View Details
Dexlansoprazole 99%View Details -
 Dexlansoprazole 98%View Details
Dexlansoprazole 98%View Details -
 Dexlansoprazole 98%View Details
Dexlansoprazole 98%View Details -
 138530-94-6 Dexlansoprazole 99%View Details
138530-94-6 Dexlansoprazole 99%View Details
138530-94-6 -
 Dexlansoprazole 98%View Details
Dexlansoprazole 98%View Details -
 Dexlansoprazole 98%View Details
Dexlansoprazole 98%View Details -
 Dexlansoprazole 98% (HPLC) CAS 138530-94-6View Details
Dexlansoprazole 98% (HPLC) CAS 138530-94-6View Details
138530-94-6 -
 Dexlansoprazole CAS 138530-94-6View Details
Dexlansoprazole CAS 138530-94-6View Details
138530-94-6