strontium nitrite
- CAS NO.:13470-06-9
- Empirical Formula: N2O4Sr
- Molecular Weight: 179.631
- EINECS: 236-731-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
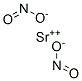
What is strontium nitrite?
Description
Strontium nitrite has the formula of Sr(NO2)2 and the
molecular weight of 179.6311 g/mol. It can be made by
the usual double decomposition reaction of sodium
nitrite and SrCl2. It forms a tetrahydrate which is
isomorphous with the calcium and barium salts:
Na2NO2 (aq) + SrCl2 ? SrNO2 (aq) + 2NaCl (aq)
If strontium nitrite is added to a mixture of magnesium–
strontium nitrate, normally used in pyrotechnic
formulations, it prevents aging of the nitrate mixture
in the presence of water until usage in fireworks. Studies
by isothermal microcalorimetry show that the addition
of strontium nitrite to a 50% magnesium–50% strontium
nitrate composition eliminated the induction reaction
normally observed in closed ampoule studies in air at
50 C and relative humidity in the range 65–69%.
Chemical properties
white or yellowish powder(s); hygroscopic needles [HAW93]
Physical properties
Anhydrous strontium nitrite, Sr(NO2)2, has the CAS
number of 13780-06-8 and occurs as yellow-white crystals.
It is very hygroscopic. Its density is 2.23 g/cm3
and it decomposes at >650°C to form nitrogen oxides
and SrO. It is slightly soluble in alcohol (0.04 g/100 ml
at 20°C) but soluble in water at 72.1 g/100 ml at 20°C.
Sr(NO2)2·4H2O loses two waters of hydration at about
80°C and one at 105°C to form the monohydrate. It loses
the other at about 155°C. The anhydrate, if further heated,
is unstable and oxidizes to the nitrate in air above 240°C:
Sr(NO2)2 + O2 + heat0Sr(NO3)2
Strontium nitrite monohydrate, Sr(NO2)2·H2O, has
the CAS number of 13470-06-9 and a molecular
weight of 197.6464 g/mol. It occurs as yellow-white
crystals which are hygroscopic. Its density is 2.84 g/
cm3. In air, the crystals begin to decompose at about 245 °C. It is soluble in water at about 58.5 g/100 ml
at 20 °C.
Properties of strontium nitrite
| Melting point: | decomposes at 240℃ [HAW93] |
| Density | 2.800 |
| solubility | soluble in H2O |
| form | white-yellow hygroscopic needles |
| color | white-yellow hygroscopic
needles |
| Water Solubility | g/100g solution H2O: 43.1 (35°C), 58.1 (98°C); solid phase, Sr(NO2)2 ·H2O [KRU93]; insoluble alcohol [HAW93] |
| CAS DataBase Reference | 13470-06-9 |
| EPA Substance Registry System | Nitrous acid, strontium salt (13470-06-9) |
Safety information for strontium nitrite
Computed Descriptors for strontium nitrite
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
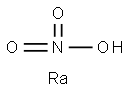
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1