Barium nitrate
Synonym(s):Barium nitrate
- CAS NO.:10022-31-8
- Empirical Formula: BaN2O6
- Molecular Weight: 261.34
- MDL number: MFCD00003442
- EINECS: 233-020-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
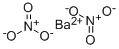
What is Barium nitrate?
Description
Barium nitrate is a stable, strong oxidiser. It is incompatible with combustible material, reducing agents, acids, acid anhydrides, and moisture-sensitive substance. Barium nitrate is poisonous, is a respiratory irritant, and is hazardous if mixed with flammable materials. Barium oxide plus zinc, aluminium and magnesium alloys are combustibles (paper, oil, wood), acids, and oxidisers and is hazardous. Mixtures with finely divided aluminium–magnesium alloys are easily ignitable and extremely sensitive to friction or impact. Barium nitrate mixed with aluminium powder, a formula for flash powder, is highly explosive. However, barium nitrate is noncorrosive in presence of glass. It is used in military thermite grenades, in the manufacturing process of barium oxide, in the vacuum tube industry, and in pyrotechnics for green flame.

Chemical properties
Barium nitrate is a shiny, white crystalline solid. It forms white crystals that are soluble in water at 20℃. It is formed by the reaction of barium carbonate or barium hydroxide with nitric acid. It is hazardous as magnesium plus barium oxide plus zinc, aluminum and magnesium alloys, combustibles (paper, oil, wood), acids, and oxidizers. Mixtures with nely divided aluminum-magnesium alloys are easily ignitable and extremely sensitive to friction or impact. Barium nitrate on contact with combustible materials will ignite. Barium nitrate mixed with aluminum powder, a formula for l ash powder is highly explosive.
Physical properties
Barium nitrate has the molecular formula of Ba(NO3)2 and the molecular weight of 261.3745 g/mol. It is also known as “nitrobarite”. Its CAS number is 10022-31-8. It is soluble in water.
It can be prepared by a number of methods. The reaction between nitric acid and barium metal is one way and reaction with BaO or BaCO3 is another. Barium hydroxide and ammonium nitrate also form the product but ammonia is released as a by-product:
2HNO3 + Ba ---> Ba(NO3)2 +H2
2HNO3 + BaO ---> Ba(NO3)2 +H2O
Ba(OH)2 + 2NH4NO3 ---> Ba(NO3)2 + 2NH3 + 2H2O
Barium nitrate can also be prepared by the reaction of barium carbonate or barium carbonate with nitric acid:
BaCO3 + 2HNO3 ---> Ba(NO3)2 + CO2 +H2O
The Uses of Barium nitrate
Barium nitrate is used in industry in the production of green signal lights, to remove gases from vacuum tubes, and in the production of barium oxide.
The Uses of Barium nitrate
manufacture of BaO2; pyrotechnics for green fire; green signal lights; in the vacuum-tube industry.
The Uses of Barium nitrate
Barium nitrate [Ba(NO3)2] burns with a bright green flame and is used in signal flares and pyrotechnics. It can be produced by treating barium carbonate with nitric acid.
What are the applications of Application
Barium nitrate is a Ba2+ salt with a range of industrial applications
Preparation
Barium Nitrate can be prepared by a number of methods. The reaction
between nitric acid and barium metal is one way
and reaction with BaO or BaCO3 is another. Barium
hydroxide and ammonium nitrate also form the product
but ammonia is released as a by-product:
2HNO3+ Ba→Ba(NO3)2+H2
2HNO3+ BaO→Ba(NO3)2+H2O
Ba(OH)2+ 2NH4NO3→Ba(NO3)2+ 2NH3+ 2H2O
Barium nitrate can also be prepared by the reaction of
barium carbonate or barium carbonate with nitric acid:
BaCO3+ 2HNO3→Ba(NO3)2+ CO2+H2O
In this method, barium carbonate is suspended in
nitric acid. The solution is filtered and the product
crystallizes out. Alternatively, barium carbonate and
nitric acid are added to a saturated solution of
barium nitrate. The product is then obtained by crystallization.
Barium nitrate may also be prepared by adding
sodium nitrate to a saturated solution of barium
chloride. Barium nitrate precipitates out from the
solution. The precipitate is filtered, washed with
alcohol and dried.
Definition
ChEBI: Barium nitrate is an inorganic nitrate salt of barium. It is an inorganic barium salt and an inorganic nitrate salt.
General Description
A white crystalline solid. Noncombustible, but accelerates burning of combustible materials. If large quantities are involved in fire or the combustible material is finely divided, an explosion may result. May explode under prolonged exposure to heat or fire. Toxic oxides of nitrogen produced in fires.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Mixtures of metal nitrates with alkyl esters may explode, owing to the formation of alkyl nitrates. Mixtures of nitrates with phosphorus, tin(II) chloride, or other reducing agents may react explosively [Bretherick 1979 p. 108-109].
Hazard
Strong oxidizing agent. See barium.
Health Hazard
Exposures to barium nitrate by ingestion or inhalation cause poisoning. The symptoms include, but are not limited to, ringing of the ears, dizziness, irregular and elevated blood pressure, blurred vision, irritation to the respiratory system, tightness of muscles (especially in the face and neck), vomiting, diarrhea, abdominal pain, muscular tremors, anxiety, weak- ness, labored breathing, cardiac irregularity, and convulsions. Poisoned workers also suffer from kidney damage, cardiac or respiratory failure, tremors, convulsions, coma, and possi- bly death. Prolonged periods of exposure to barium nitrate is known to cause damage of the liver (anemia and possibly methemoglobinemia), spleen, kidney, bone marrow, and the CNS.
Flammability and Explosibility
Non flammable
Safety Profile
A poison by ingestion, subcutaneous, parenteral, and intravenous routes. An irritant to slun and eyes. When heated to decomposition it emits very toxic fumes of NO,. An oxiduer. Mixtures with finely divided aluminum-magnesium alloys are easily ignitable and extremely sensitive to friction or impact. Such mixtures are used in chemical photoflash applications. Incompatible with (Mg + BaO2 + Zn), Al, and Mg alloys. When heated to decomposition it emits toxic fumes of NO,. See also BARIUM COMPOUNDS (soluble) and NITRATES.
Potential Exposure
Barium nitrate is used in making fireworks (in green fire pyrotechnics), signal lights, ceramics; and in the electronics industry; to make BaO2. Once used in the vacuum tube industry.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Seealso First Aid section in “Barium” entry.
Storage
Barium nitrate should be kept stored in a tightly closed container, in a cool, dry, venti- lated area, protected against physical damage. It should be separated from heat, sources of ignition, incompatible substances, combustibles, and organic or other readily oxidizable materials. Barium nitrate should not be stored on wood l oors or with food and beverages
Shipping
UN1446 Barium nitrate, Hazard Class: 5.1; Labels: 5.1—Oxidizer, 6.1—Poisonous materials.
Purification Methods
Crystallise it twice from water (4mL/g) and dry it overnight at 110o. It decomposes at higher temperatures to give mostly the oxide and the peroxide with only a little of the nitrite. POISONOUS. [Ehrlich in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 941 1963.]
Incompatibilities
A strong oxidizer. Decomposes in heat; may detonate if confined in elevating temperatures. Keep away from strong acids; reducing agents. Contact with organic and combustible materials (such as wood, paper, oil and fuels); and aluminum magnesium alloys, since violent reactions occur. Contact with sulfur powder and finely divided metals can form shock-sensitive compounds.
Waste Disposal
Dissolve waste in 6-MHCl. Neutralize with NH4OH. Precipitate with excess sodium carbonate. Filter, wash and dry precipitate and return to supplier.
Precautions
After accidental exposures to barium nitrate by ingestion, swallow, or inhalation, workers should induce vomiting immediately as directed by medical personnel. Never give any- thing by mouth to an unconscious person. Get medical attention immediately
Properties of Barium nitrate
| Melting point: | 592 °C (dec.)(lit.) |
| Density | 3.23 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 94g/l |
| form | Solid |
| color | White |
| Specific Gravity | 3.24 |
| Odor | odorless |
| PH | 5.0-8.0 (50g/l, H2O, 25℃) |
| Water Solubility | 9 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,983 |
| Solubility Product Constant (Ksp) | pKsp: 2.33 |
| Exposure limits | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| Dielectric constant | 5.8(0.0℃) |
| Stability: | Stable. Strong oxidizer - contact with combustible material may lead to fire. Incompatible with combustible material, reducing agents, acids, acid anhydrides. Moisture sensitive. |
| CAS DataBase Reference | 10022-31-8(CAS DataBase Reference) |
| EPA Substance Registry System | Barium nitrate (10022-31-8) |
Safety information for Barium nitrate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H301:Acute toxicity,oral H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Barium nitrate
| InChIKey | IWOUKMZUPDVPGQ-UHFFFAOYSA-N |
Barium nitrate manufacturer
Zama Chemical
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Barium nitrate CAS 10022-31-8View Details
Barium nitrate CAS 10022-31-8View Details
10022-31-8 -
 Barium nitrate CAS 10022-31-8View Details
Barium nitrate CAS 10022-31-8View Details
10022-31-8 -
 Barium nitrate CAS 10022-31-8View Details
Barium nitrate CAS 10022-31-8View Details
10022-31-8 -
 Barium nitrate CAS 10022-31-8View Details
Barium nitrate CAS 10022-31-8View Details
10022-31-8 -
 Barium nitrate CAS 10022-31-8View Details
Barium nitrate CAS 10022-31-8View Details
10022-31-8 -
 Barium nitrate CAS 10022-31-8View Details
Barium nitrate CAS 10022-31-8View Details
10022-31-8 -
 Barium CAS 10022-31-8View Details
Barium CAS 10022-31-8View Details
10022-31-8 -
 Barium CAS 10022-31-8View Details
Barium CAS 10022-31-8View Details
10022-31-8
