CALCIUM NITRIDE
Synonym(s):Tricalcium dinitride
- CAS NO.:12013-82-0
- Empirical Formula: Ca3N2
- Molecular Weight: 148.25
- MDL number: MFCD00015985
- EINECS: 234-592-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
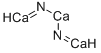
What is CALCIUM NITRIDE?
Description
Calcium nitride has the formula of Ca3N2 and the
molecular weight of grams per mole. It can be prepared
by the reactionofCametal inapure streamof nitrogen gas:
3Ca + 2N2 ? Ca3N2
A second method is the decomposition of an amide:
3Ca(NH2)2 + heat ? Ca3N2 + 4NH3
This must be accomplished in an inert atmosphere
(not nitrogen gas). It is a reddish-brown powder. Its
CAS number is 12013-82-0 and its density is 2.76 g/
cm3. It melts at 1195 °C (2183 F).α-Calcium nitride
is the commonly encountered form. It has an antibixbyite
structure similar to Mn2O3, except that the
positions of the ions are reversed: calcium (Ca2+)
ions take the oxide (O2-) positions and nitride ions
(N3-) that of the manganese (Mn3+). If Ca metal is
burned in air, it forms the oxide, CaO, and the
nitride, Ca3N2. The latter reacts with atmospheric
moisture to produce the hydroxide of calcium and
ammonia:
Ca3N2 + 6H2O ? 3Ca(OH)2 + 2NH3
Chemical properties
12mm pieces and smaller, -200 mesh 99% pure; brown crystal(s); hex: a=0.3533nm, c=0.411 nm; cub: a=1.138 nm [CIC73] [CER91]
The Uses of CALCIUM NITRIDE
Recent advances in the utilization of single crystals for a wide variety of purposes, especially in various electronic surveillance and detection applications, has generated considerable research in the development of improved techniques for growing single crystalline materials. A problem has arisen, however, in that many of the methods relied upon heretofore fail to growcrystals having a reproducibly high quality. Calcium nitride as a high temperature solvent has been used in the recrystallization of refractory aluminum nitride, that is growing single crystals of aluminum nitride from a molten solution containing the refractory material as the solute, and calcium nitride as the solvent. Crystallization is accomplished by the precipitation or crystallization of the solute, the solute being the desired crystal, from the molten solution.
The Uses of CALCIUM NITRIDE
Calcium nitride is used in the production of light-emitting diode (LED) phosphors.
The Uses of CALCIUM NITRIDE
Calcium nitride is used as a nitride source in metathesis reactions to produce complex nitrides as well as SiAlON-related optical materials.
Properties of CALCIUM NITRIDE
| Melting point: | 1195°C |
| Boiling point: | 1195 °C |
| Density | 2.63 g/mL at 25 °C(lit.) |
| solubility | soluble in H2O, acid solutions; insoluble in ethanol |
| form | Pieces |
| color | Brown |
| Specific Gravity | 2.63 |
| Water Solubility | Insoluble in water. |
| Sensitive | Moisture Sensitive |
| CAS DataBase Reference | 12013-82-0(CAS DataBase Reference) |
| EPA Substance Registry System | Calcium nitride (Ca3N2) (12013-82-0) |
Safety information for CALCIUM NITRIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for CALCIUM NITRIDE
| InChIKey | DVJMVZQXJNJSJR-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Calcium nitride CAS 12013-82-0View Details
Calcium nitride CAS 12013-82-0View Details
12013-82-0 -
 Calcium nitride CAS 12013-82-0View Details
Calcium nitride CAS 12013-82-0View Details
12013-82-0 -
 Calcium nitride CAS 12013-82-0View Details
Calcium nitride CAS 12013-82-0View Details
12013-82-0 -
 Calcium nitride CAS 12013-82-0View Details
Calcium nitride CAS 12013-82-0View Details
12013-82-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
