STRONTIUM NITRIDE
Synonym(s):Tristrontium dinitride
- CAS NO.:12033-82-8
- Empirical Formula: N2Sr3
- Molecular Weight: 290.87
- MDL number: MFCD00054068
- EINECS: 234-795-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
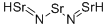
What is STRONTIUM NITRIDE?
Description
Strontium nitride should have the molecular formula
of Sr3N2 and the molecular weight of 290.8752 g/mol.
However, according to some sources, Strontium does
not form the ionic compound Sr3N2 and Sr2N is the
only compound that has been properly characterized
to date. The reaction of Sr metal in a pure stream of
nitrogen gas produces the “subnitride”:
4Sr +N2 ? 2Sr2N
A second method would be the decomposition of an
amide:
2Sr(NH2)2 + Heat ? 2Sr2N+ 2NH3 +H2
Physical properties
In air, it strontium metal burns and produces strontium
oxide and strontium nitride. But, since it does not
react with nitrogen below 380 °C, it will only form the
oxide spontaneously at room temperature. To produce
the pure nitride, it is necessary to maintain the temperature
above 380 °C in air or to heat the metal in pure
nitrogen gas. In air, the molten mass, as it cools from
380 °C, tends to convert some of the nitride to oxide.
Strontium nitride is described as Sr3N2,produced by burning strontium metal in air (resulting in a mixture with strontium oxide) or in nitrogen. Like other alkaline earth metal nitrides, it reacts with water to give strontium hydroxide and ammonia: Sr3N2 + 6H2O 3Sr(OH)2 + 2NH3
The Uses of STRONTIUM NITRIDE
Strontium nitride (Sr3N2) can be used in the synthesis of ternary nitride oxide with molybdenum nitride under a low partial pressure of oxygen. It can also be used in the synthesis of barium-strontium silicate-based hollow structures for potential usage in drug delivery, catalysis, and sensors.
Properties of STRONTIUM NITRIDE
| Melting point: | 1030℃ [CIC73] |
| solubility | reacts with H2O; soluble in HCl |
| form | Powder |
| color | tan |
| Sensitive | moisture sensitive |
| CAS DataBase Reference | 12033-82-8 |
| EPA Substance Registry System | Strontium nitride (Sr3N2) (12033-82-8) |
Safety information for STRONTIUM NITRIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for STRONTIUM NITRIDE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Strontium nitride CAS 12033-82-8View Details
Strontium nitride CAS 12033-82-8View Details
12033-82-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
