Beryllium nitrate
Synonym(s):Beryllium nitrate solution
- CAS NO.:13597-99-4
- Empirical Formula: BeN2O6
- Molecular Weight: 133.02
- MDL number: MFCD00046184
- EINECS: 237-062-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
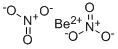
What is Beryllium nitrate?
Description
Beryllium nitrate is said to have the molecular
formula of Be(NO3)2 and the molecular weight of grams
per mole. It is very soluble in water.
It could be prepared by the action of nitric acid on
beryllium carbonate, oxide or hydroxide:
BeCO3 + 2HNO3 ? Be(NO3)2 + CO2 +H2O
Be(OH)2 + 2HNO3 ? Be(NO3)2 + 2H2O
It is described as Be(NO3)2·4H2O, a compound that
forms colorless, deliquescent crystals that are soluble in
water, and used to introduce beryllium oxide into materials used in incandescent mantles. However, in
view of recent developments concerning the behavior
of the Be3+ cation in solution, the more likely reactions
are:
BeCO3 + 2HNO3 ? Be(OH)NO3 + CO2 + 1/2H2O
Be(OH)2 + 2HNO3 ? Be(OH)NO3 + 3/2H2O
Chemical properties
White to faintly yellowish, deliquescent mass. Soluble in water, alcohol.
Physical properties
DTA analysis of the tetrahydrate showed that it
dissolves in its own waters of hydration at about
60.4°C. Heating above 101°C causes decomposition
with loss of water and oxides of nitrogen plus formation
of the oxide. The decomposition is complete at 250°C.
Thermal decomposition of these salt hydrates occurs
by release of nitric acid which then breaks down to
form the nitrogen oxides, rather than break down of
the nitrate anion itself. The anhydrate has been prepared
by the reaction of beryllium chloride with N2O4 dissolved
in ethylacetate. If heated in a vacuum above
50°C, the product, Be(NO3)2·2N2O4, evolves oxides of
nitrogen to produce the anhydrate.
The tetrahydrate has a CAS number of 13597-99-4 and
molecular weight is grams per mole. Anhydrous beryllium
nitrate is a white to pale yellow crystalline solid.
Beryllium nitrate trihydrate (CAS number = 7787-55-5)
forms slightly yellow crystals with a vapor pressure
49.8 mm Hg at 25°C. Its melting point is 60.5°C and it
decomposes completely at 102.3°C. Its Enthalpy of
vaporization = 37.72 kJ/mol and its boiling point is
83°C at 760 mm Hg where it becomes anhydrous. Beryllium
nitrate is a tumorigen, mutagen and reproductive
effector in rats and, presumably in humans.
Beryllium metal dissolves readily in nonoxidizing
acids such as HCl and H2SO4, but not in nitric acid as this forms the oxide (this behavior is similar to that of
aluminum metal). Beryllium, again similarly to
aluminum, dissolves in warm alkali to form the beryllate
anion, Be(OH)42-, and hydrogen gas.
Physical properties
Beryllium nitrate, also known as beryllium dinitrate, is an ionic beryllium salt of nitric acid with the chemical formula Be(NO).Each formula unit is composed of one Be2+ cation and two NO-anions.
The Uses of Beryllium nitrate
Beryllium nitrate is used as a hardening agent for mantles on gas lanterns.
The Uses of Beryllium nitrate
Chemical reagent, gas-mantle hardener.
Preparation
Anhydrous beryllium nitrate has been prepared by the reaction of anhydrous beryllium chloride with an ethylacetate-dinitrogen tetroxide mixture to give Be(NO3)2 · 2N2O4 [Addison and Walker 1963]. When heated above 50 °C (120 °F) in a vacuum, oxides of nitrogen are evolved, leaving anhydrous beryllium nitrate. The anhydrous salt is stable to approximately 125 °C (255 °F), when a sudden decomposition occurs to give nitrogen tetroxide and a volatile compound that separates from the gas phase as colorless Be4O(NO3)6 crystals. These crystals are slowly hydrolyzed when added to water.
Hazard
Very toxic. Oxidizing material, dangerous fire risk.
Hazard
Beryllium nitrate is a toxic chemical,like all other beryllium compounds. It is also an irritant in small doses. When burned, it gives off irritating or toxic fumes. However, when massive short-term exposure occurs, acute pneumonitis can set in, but symptoms do not manifest themselves for 3 days.
Safety Profile
Confirmed carcinogen. Poison byintraperitoneal, intravenous, and subcutaneous routes.Experimental reproductive effects. When heated todecomposition it emits very toxic fumes of BeO and NOx.
Synthesis
Beryllium nitrate can be prepared by reacting beryllium hydroxide in nitric acid.
Be(OH) + 2 HNO → Be(NO) + 2 HO
Properties of Beryllium nitrate
| Melting point: | ~60℃ [MER06] |
| Boiling point: | decomposes at 100–200℃ [HAW93] |
| Density | 1.02 g/mL at 20 °C |
| storage temp. | Store at +15°C to +25°C. |
| solubility | soluble in ethanol |
| form | Liquid |
| color | Clear colorless |
| PH | 0.5 (H2O, 20°C) |
| Water Solubility | g Ba(NO3)2/100g H2O: 97 (0°C), 108 (20°C), 178 (60°C); soluble alcohol [LAN05] [HAW93] |
| Merck | 13,1174 |
| CAS DataBase Reference | 13597-99-4 |
| EPA Substance Registry System | Beryllium nitrate (Be(NO3)2) (13597-99-4) |
Safety information for Beryllium nitrate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H301:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H372:Specific target organ toxicity, repeated exposure H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P220:Keep/Store away from clothing/…/combustible materials. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Beryllium nitrate
Beryllium nitrate manufacturer
ARRAKIS INDUSTRIES LLP
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
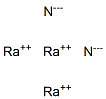
You may like
-
 13597-99-4 BERYLLIUM NITRATE 99%View Details
13597-99-4 BERYLLIUM NITRATE 99%View Details
13597-99-4 -
 Beryllium standard solution CASView Details
Beryllium standard solution CASView Details -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1