RADIUMNITRATE
- CAS NO.:10213-12-4
- Empirical Formula: H3NO3Ra
- Molecular Weight: 291.03
- Update Date: 2023-10-18 15:15:00
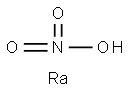
What is RADIUMNITRATE?
Description
Radium nitrate had the molecular formula of
Ra(NO3)2 and the molecular weight of 350.0343 g/mol.
It can be prepared by a number of methods. The reaction
between nitric acid and radium metal is one way and
reaction with Ra RaO or RaCO3 is another. Radium
hydroxide and ammonium nitrate also form the product
but ammonia is released as a by-product:
2HNO3 + Ra ? Ra(NO3)2 +H2
2HNO3 + RaO ? Ra(NO3)2 +H2O
Ra(OH)2 + 2NH4NO3 ? Ra(NO3)2 + 2NH3 + 2H2O
Radium nitrate can also be prepared by the reaction
of radium carbonate with nitric acid. The product is
soluble but can be isolated by evaporation of the solution
at low temperature.
Preparation
Radium nitrate can also be prepared by the reaction
of radium carbonate with nitric acid. The product is
soluble but can be isolated by evaporation of the solution
at low temperature. It occurs as the anhydrate,
like barium nitrate, but is slightly more soluble at
12.1 g/100 ml at 20°C. Its CAS number is 10213-12-4.
When heated, Ra(NO3)2 decomposes at about 280°C to
form RaO. A number of other oxides have been
proposed but this decomposition mechanism has not
been thoroughly studied. Like the barium analog, the
decomposition is probably complex and may involve
the peroxides as well. Temperatures above 820°C are
probably required to obtain an oxide. Whether the nitrite
is involved during the decomposition is not known.
Little interest has been shown for this compound in
industry. The main academic and scientific interest in
radium nitrate has been the fact that, since it is soluble
(and the 226Ra isotope has a 1600 year half-life), the presence
of radium nitrate, even in pico-gram quantities
(10-12 g/ml) of ingested water is sufficient to cause
health problems in the human body because it accumulates
in the bones while continuing to emit energetic
g-rays.
Properties of RADIUMNITRATE
| color | crystals, crystalline |
| Water Solubility | 13.9 |
Safety information for RADIUMNITRATE
Computed Descriptors for RADIUMNITRATE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1