Strontium fluoride
Synonym(s):Strontium difluoride;Strontium difluoride (SrF2 )
- CAS NO.:7783-48-4
- Empirical Formula: F2Sr
- Molecular Weight: 125.62
- MDL number: MFCD00011251
- EINECS: 232-000-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57

What is Strontium fluoride?
Chemical properties
White powder. Soluble in hydrochloric acid and hydrogen fluoride; insoluble in water.
Chemical properties
Strontium fluoride, also called strontium difluoride and strontium(II) fluoride, is a fluoride of strontium. It is a stable brittle white crystalline solid. It forms face-centered cubic crystals, with structure of calcium fluoride. It is almost insoluble in water. Strontium fluoride is prepared by reaction of strontium chloride with fluorine gas, by action of hydrofluoric acid on strontium carbonate, or by addition of potassium chloride to strontium chloride solution. It irritates eyes and skin, and is harmful when inhaled or ingested.
Physical properties
Strontium fluoride, SrF2, also called "strontium
difluoride" and "strontium (II) fluoride", is a stable, brittle, white crystalline solid with a melting point of 1477°C and a boiling point of 2460°C. Its molecular weight is 125.62 g/mol. Its density is 4.24 g/cm3. Its melting point has also been given as 1463°C and a boiling point of 2489°C. It is virtually insoluble since its solubility in water is 0.00012 g/100 ml of water at 18°C.
SrF2 is almost insoluble in water (its KSP value is
approximately 4.33×109 at 25°C. It irritates
eyes and skin, and is harmful when inhaled or
ingested.
The Uses of Strontium fluoride
Strontium fluoride is transparent to light in the wavelengths from the vacuum UV (150 nm) to the near IR (11 mm). Its optical properties are intermediate to CaF2 and BaF2. Strontium fluoride is used as an optical material for a small range of special applications, for example, as an optical coating on lenses and also as a thermoluminescent dosimeter crystal. Another use is as a carrier of the 90Sr radioisotope in thermoelectric generators. SrF2 is also used as a single crystal for lasers.
The Uses of Strontium fluoride
Substitute for other fluorides, electronic and optical applications, single crystals for lasers, hightemperature dry-film lubricants.
The Uses of Strontium fluoride
Strontium fluoride is used as an optical material transparent from vacuum ultraviolet (150 nm) to infrared (11 μm) for a small range of special applications. Its optical properties are intermediate to calcium fluoride and barium fluoride. It is also used as an optical coating on lenses.
Preparation
Strontium fluoride is synthesized by the fluorination of strontium dichloride, or by action of
hydrofluoric acid on strontium carbonate:
SrCl2 (aq)+ F2 (gas)→SrF2 (solid)+ Cl2 (gas)
SrCO3+ 2HF→SrF2+ CO2+H2O
Note that the first reaction depends upon the
insolubility of SrF2 in water. Strontium fluoride is
a water-insoluble source for use in oxygen-sensitive applications, such as metal production since it in
nonhygroscopic.
Hazard
Toxic material.
Flammability and Explosibility
Not classified
Safety Profile
Moderately toxic by intravenous route. Mildly toxic by ingestion. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES and STRONTIUM COMPOUNDS.
Properties of Strontium fluoride
| Melting point: | >1400 °C (lit.) |
| Boiling point: | 2489 °C (lit.) |
| Density | 4.24 g/mL at 25 °C (lit.) |
| refractive index | 1.442 |
| solubility | soluble in dilute acid solutions |
| form | powder |
| color | White |
| Specific Gravity | 4.24 |
| Odor | odorless |
| Water Solubility | 11.7 mg/100 mL (18 ºC) |
| Merck | 14,8842 |
| Solubility Product Constant (Ksp) | pKsp: 8.36 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Dielectric constant | 6.4699999999999998 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 7783-48-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Strontium fluoride(7783-48-4) |
| EPA Substance Registry System | Strontium fluoride (SrF2) (7783-48-4) |
Safety information for Strontium fluoride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Strontium fluoride
Strontium fluoride manufacturer
Madras Fluorine Private Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
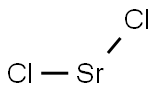

You may like
-
 Strontium fluoride 98%View Details
Strontium fluoride 98%View Details -
 Strontium Fluoride CAS 7783-48-4View Details
Strontium Fluoride CAS 7783-48-4View Details
7783-48-4 -
 Strontium Fluoride CAS 7783-48-4View Details
Strontium Fluoride CAS 7783-48-4View Details
7783-48-4 -
 Strontium Fluoride CAS 7783-48-4View Details
Strontium Fluoride CAS 7783-48-4View Details
7783-48-4 -
 Strontium fluoride CAS 7783-48-4View Details
Strontium fluoride CAS 7783-48-4View Details
7783-48-4 -
 Strontium fluoride CAS 7783-48-4View Details
Strontium fluoride CAS 7783-48-4View Details
7783-48-4 -
 Strontium fluoride CAS 7783-48-4View Details
Strontium fluoride CAS 7783-48-4View Details
7783-48-4 -
 Strontium fluoride 95% CAS 7783-48-4View Details
Strontium fluoride 95% CAS 7783-48-4View Details
7783-48-4