Fluorinated carbon
- CAS NO.:11113-63-6
- Empirical Formula: CH4F2
- Molecular Weight: 54.04
- MDL number: MFCD00243019
- EINECS: 234-345-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-13 11:09:07
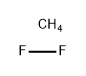
What is Fluorinated carbon?
Description
Graphite fluoride is one of the research hotspots of new high-tech, high-performance and high-efficiency carbon/graphite materials in the world. With its excellent performance and unique quality, it is a wonderful flower in the family of functional materials.
Chemical properties
-200 mesh, 99.9% pure; polymer [ALD94] [CER91]
Chemical properties
1. Good chemical resistance. Chemical resistance is better than PTFE at room temperature. It is also stable to concentrated sulfuric acid and concentrated nitric acid, and insoluble in various organic solvents. However, hot concentrated sulfuric acid and hot alkali have certain invasion to it, and react with alkali metals and alkali halide compounds at high temperature to form fluorinated alkali and amorphous carbon.
2. Good thermal stability (only a small amount of weight loss is higher than 500 °C).
3. Good lubricating performance, the lowest friction coefficient among greases and solid lubricants (molybdenum disulfide, graphite, etc.)
4. Good electrical insulation, (CF)n resistivity is above 3×103Ω/cm.
5. The Mohs hardness is 1.0 to 2.06, and the particle size distribution is very wide, ranging from less than 1 μm to more than several millimeters.
The Uses of Fluorinated carbon
Lithium anode batteries, lubricant chromatographic materials.
Definition
Fluorinated graphite cathode material.
Manufacturing Process
In the reaction system of graphite and fluorine, trace metal fluorides (LiF, MgF2, AlF3 and CuF2) are added as catalysts, and the synthesis can be carried out below 300 °C. Due to the addition of trace metal fluorides, the properties of graphite fluoride are changed, and the electrical conductivity is increased by an order of magnitude. The purity of graphite and fluorine gas is required to be above 99.4%, and the purity of catalyst is more than 98%.
Properties of Fluorinated carbon
| Melting point: | 300°C (dec.) |
| CAS DataBase Reference | 11113-63-6 |
| EPA Substance Registry System | Fluorine, compd. with graphite (11113-63-6) |
Safety information for Fluorinated carbon
Computed Descriptors for Fluorinated carbon
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
