Caesium fluoride
Synonym(s):B2T;BM600;CSF;LAMC2;NSC 84270
- CAS NO.:13400-13-0
- Empirical Formula: CsF
- Molecular Weight: 151.9
- MDL number: MFCD00010960
- EINECS: 236-487-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
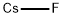
What is Caesium fluoride?
Description
Cesium fluoride (CsF) has higher solubility than NaF or KF. Cesium is less electronegative than sodium or potassium and is larger in size, both properties which allow for easier dissociation from the fluorine ion. Cesium fluoride is less hygroscopic than other sources of highly dissociated fluorine such as TBAF. The moderately basic nature of fluorine also makes it useful as a base in certain reactions, especially when a non-nucleophilic base is desired.
Chemical properties
white crystalline powder
The Uses of Caesium fluoride
A preperation of building block for synthesis of fluoroallylic compounds.
The Uses of Caesium fluoride
Catalyst in oxidation reactions; as a solid base when adsorbed onto Celite, q.v. Fluoride source in organic synthesis. Inorganic scintillator.
The Uses of Caesium fluoride

To a solution of the bromide (1.2 g, 4.7 mmol) in dry DMF (36 mL) degassed with N2 was added Pd(PPh3)4 (270 mg, 0.23 mmol) and the stannane (1.85 g, 5.6 mmol). The mixture was flushed with N2 for 5 min, then stirred at 80 C overnight. After cooling, the mixture was partitioned between EtOAc and brine. The org layer was washed with brine (2x), dried, and concentrated to give 3.5 g of a yellow oil. The material was purified by Prep LC (80 g silica, 5-10% EtOAc/heptane) to provide the product as a colorless oil. [900 mg, 88%]
The Uses of Caesium fluoride
Used as a base in a Suzuki cross-coupling synthesis of ortho-substituted biaryls.5 Also employed as a reagent for nucleophilic fluorination of primary halides and sulfonates in protic media such as tert-butyl and tert-pentyl alcohols.6
The Uses of Caesium fluoride
Cesium fluoride is an inorganic compound with the chemical formula CsF, which is a hygmoscopic white salt. Cesium fluoride can be used as a source of fluoride anion for organic synthesis. Caesium has the highest electropositivity of all non-radioactive elements and fluorine has the highest electronegativity of all elements.
What are the applications of Application
Cesium fluoride is a preperation of building block for synthesis of fluoroallylic compounds
General Description
Cesium fluoride is an inorganic compound known to be a source of fluoride ion and a catalyst in organic synthesis. It has been used in many organic reactions like 1,4?elimination, desilylation, transesterification, acylation, nucleophilic aromatic substitution, etherification, cross?coupling reactions and so on.
Hazard
A poison.
Flammability and Explosibility
Not classified
Safety Profile
A poison. Incompatible with benzenediazonium tetrafluoroborate and difluoroamine. When heated to decomposition it emits toxic fumes of F-.
Purification Methods
Crystallise it from aqueous solution by adding ethanol.
Properties of Caesium fluoride
| Melting point: | 682 °C(lit.) |
| Boiling point: | 1251 °C |
| Density | 4.115 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 25℃ |
| Flash point: | 1251°C |
| storage temp. | 0-6°C |
| solubility | H2O: 3 M at 20 °C, clear, colorless |
| form | Powder/Lump |
| appearance | White solid |
| color | White |
| Specific Gravity | 4.115 |
| PH | 6.5-7.5 (25℃, 3M in H2O) |
| Water Solubility | 369 g/100 mL (18 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.05 |
| Merck | 14,2012 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 13400-13-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Cesium fluoride(13400-13-0) |
| EPA Substance Registry System | Cesium fluoride (CsF) (13400-13-0) |
Safety information for Caesium fluoride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation H373:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Caesium fluoride
| InChIKey | XJHCXCQVJFPJIK-UHFFFAOYSA-M |
Caesium fluoride manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Cesium fluoride CAS 13400-13-0View Details
Cesium fluoride CAS 13400-13-0View Details
13400-13-0 -
 Cesium fluoride CAS 13400-13-0View Details
Cesium fluoride CAS 13400-13-0View Details
13400-13-0 -
 Cesium fluoride CAS 13400-13-0View Details
Cesium fluoride CAS 13400-13-0View Details
13400-13-0 -
 Cesium fluoride CAS 13400-13-0View Details
Cesium fluoride CAS 13400-13-0View Details
13400-13-0 -
 Cesium fluoride CAS 13400-13-0View Details
Cesium fluoride CAS 13400-13-0View Details
13400-13-0 -
 Cesium fluoride CAS 13400-13-0View Details
Cesium fluoride CAS 13400-13-0View Details
13400-13-0 -
 Cesium fluoride CAS 13400-13-0View Details
Cesium fluoride CAS 13400-13-0View Details
13400-13-0 -
 CESIUM FLUORIDE 99%View Details
CESIUM FLUORIDE 99%View Details
13400-13-0
