Sodium iodide
Synonym(s):Sodium Iodide;Sodium Iodine;Sodium monoiodide
- CAS NO.:7681-82-5
- Empirical Formula: NaI
- Molecular Weight: 149.89
- MDL number: MFCD00003532
- EINECS: 231-679-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
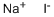
What is Sodium iodide ?
Absorption
Iodide salts are readily absorbed in the gastrointestinal tract .
Toxicity
Acute oral LD50 is 4340 mg/kg in rat and 1000 mg/kg in mouse . Chronic iodide overdoses may result in iodism, which is commonly characterized by salivation, coryza, sneezing, conjunctivitis, headache, fever, laryngitis, bronchitis, stomatitis, parotitis and oedema of the glottis . Up to 10g of sodium iodide has been administered intravenously without any signs of toxicity .
Chemical properties
White or almost white, crystalline powder or colourless crystals, hygroscopic.
Chemical properties
Sodium iodide, white solid, soluble, formed by reaction of sodium carbonate or hydroxide and hydriodic acid, and then evaporating. Used in photography, in medicine and as a source of iodide.
Physical properties
Physical Properties White crystalline deliquescent powder or granules; saline and slight bitter taste; absorbs moisture from air; slowly turns brown on exposure to air due to iodine evolved; density 3.67g/cm3; melts at 660°C; vaporizes at 1,304°C; vapor pressure 1 torr at 767°C and 5 torr at 857°C; very soluble in water, 178.7 g/100 mL at 20°C and 294 g/100 mL at 70°C; soluble in ethanol and acetone.
The Uses of Sodium iodide
Sodium iodide was used as a component in the preparation of Mayer′s hematoxylin stain solution.
It may be used in the following processes:
- Precursor in the polymerization of butyl acrylate.
- Chaotropic agent in the extraction of DNA.
- Deprotecting agent in the removal of N-tert-butyloxycarbonyl group in amino acids.
- Water-soluble fluorescence quenching reagent.
The Uses of Sodium iodide
Used as iodometric reagent
The Uses of Sodium iodide
Sodium iodide is widely used for halide exchange (Finkelstein reaction), for example in the conversion of an alkyl chloride, allyl chloride and arylmethyl chloride into their respective iodides, which are precursors for pharmaceutical and fine chemical products. They are used to ehnance the efficiency of the formation of Wittig adducts from less reactive chlorides and bromides. Appropriate prepartions find use as a nutrient supplement. Sodium iodide is used as the precursor to the control agent in ab initio emulsion polymerization. Sodium iodide finds use in the determination of dissolved oxygen in the modified Winkler method, synthesis of the fluorescent dye coppersensor-1 (CS1) for imaging labile copper pools in biological samples, and the cleavage of esters, lactones, carbamates and ethers in combination with chlorotrimethylsilane.
What are the applications of Application
Sodium iodide is a useful reactant in the Finkelstein reaction
Indications
Indicated for use as a supplement to intravenous solutions given for total parenteral nutrition (TPN). When intravenously administered for total parental nutrition, sodium iodide prevents the depletion of endogenous stores of iodine and subsequent deficiency symptoms .
Background
Sodium iodide is a compound forming white, odourless deliquescent crystals. It is a water-soluble ionic compound with a crystal lattice. Sodium iodide is a source of iodine and can be administered as a supplement for total parenteral nutrition, but it is more commonly used in veterinary medicine. It is used as an iodine supplement and expectorant. Radiolabelled compound, Iodide I-131, is used as a diagnostic tool to evaluate thyroid function and morphology.
Definition
ChEBI: A metal iodide salt with a Na(+) counterion.
Definition
sodium iodide: A white crystallinesolid, NaI, very soluble in water andsoluble in both ethanol and ethanoicacid. It is known in both the anhydrousform (cubic; r.d. 3.67; m.p.661°C; b.p. 1304°C) and as the dihydrate(monoclinic; r.d. 2.45). It is preparedby the reaction of hydrogeniodide with sodium carbonate orsodium hydroxide in solution. Likepotassium iodide, sodium iodide inaqueous solution dissolves iodine toform a brown solution containingthe I3- ion. It finds applications inphotography and is also used in medicineas an expectorant and in the administrationof radioactive iodine for studies of thyroid function and fortreatment of diseases of the thyroid.
Preparation
Sodium iodide is prepared by adding hydriodic acid or an acidic iodide solution to a solution of sodium hydroxide or sodium carbonate, followed by evaporation and crystallization: NaOH + HI → NaI + H2O
The solution is filtered to remove any impurities prior to its evaporation and crystallization.
General Description
Sodium iodide is an inorganic salt. Mixture of chlorotrimethylsilane/sodium iodide in acetonitrile is a useful reagent for the cleavage of esters, lactones, carbamates and ethers.
Safety Profile
Moderately toxic by ingestion, intravenous, and intraperitoneal routes. Human teratogenic effects by ingestion: developmental abnormalities of the endocrine system. Human reproductive effects by ingestion: effects on newborn, including postnatal measurements. A skin and eye irritant. Reacts violently with BrF3, HClO4, oxidants. When heated to decomposition it emits toxic fumes of Iand Na2O. See also IODIDES.
Purification Methods
Crystallise NaI from water/ethanol solution and dry it for 12hours under vacuum, at 70o. Alternatively, dissolve it in acetone, filter it and cool it to -20o; the resulting yellow crystals are filtered off and heated in a vacuum oven at 70o for 6hours to remove acetone. The NaI is then crystallised from very dilute NaOH, dried under vacuum, and stored in a vacuum desiccator [Verdin Trans Faraday Soc 57 484 1961].
Properties of Sodium iodide
| Melting point: | 661 °C (lit.) |
| Boiling point: | 1300 °C |
| Density | 3.66 |
| vapor density | >1 (vs air) |
| vapor pressure | 1 hPa (767 °C) |
| refractive index | 1.7745 |
| Flash point: | 1300-1304°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | water: soluble(lit.) |
| form | beads |
| appearance | White solid |
| pka | 0.067[at 20 ℃] |
| Specific Gravity | 3.67 |
| color | White |
| PH | 6-9 (50g/l, H2O, 20℃) |
| Water Solubility | 184 g/100 mL (25 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8631 |
| Exposure limits | ACGIH: TWA 0.01 ppm |
| Dielectric constant | 7.2800000000000002 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 7681-82-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium iodide(7681-82-5) |
| EPA Substance Registry System | Sodium iodide (7681-82-5) |
Safety information for Sodium iodide
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H372:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium iodide
| InChIKey | FVAUCKIRQBBSSJ-UHFFFAOYSA-M |
Sodium iodide manufacturer
Lanya Chem Industries Pvt Ltd
Svaks Biotech India Pvt Ltd
Ishita Drugs And Industries Ltd
Aspen Biopharma Labs Pvt Ltd
ARRAKIS INDUSTRIES LLP
New Products
1-Amino-1-cyclohexanecarboxylic acid 2-Hydroxyacetamide Ethyl 5-cyclopropyl-1H-pyrazole-3-carboxylate 2,4,6-Trichloroaniline ELECTROLYTIC IRON POWDER 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate N,N'-diallyl-1,3-diaminopropanedihydrochloride 3-Iodo-6-nitroindazole (4-(4-Fluorophenoxy)phenyl)methanamine Tert-butyl N-(pent-4-yn-2-yl) carbamate 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine 2-chloro-4-methyl-5-nitro pyridine 2,5-dibromopyridine terephthalonitrile 5-Bromo-4-chloro-2(1h)-pyridinone, 5-Fluoro-2-Oxindole N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA]Related products of tetrahydrofuran


You may like
-
 Sodium Iodide 98%View Details
Sodium Iodide 98%View Details -
 Sodium iodide 99%View Details
Sodium iodide 99%View Details -
 Sodium iodide hydrate CAS 7681-82-5View Details
Sodium iodide hydrate CAS 7681-82-5View Details
7681-82-5 -
 Sodium iodide hydrate CAS 7681-82-5View Details
Sodium iodide hydrate CAS 7681-82-5View Details
7681-82-5 -
 Sodium iodide hydrate CAS 7681-82-5View Details
Sodium iodide hydrate CAS 7681-82-5View Details
7681-82-5 -
 Sodium iodide 98%View Details
Sodium iodide 98%View Details -
 Sodium iodide CAS 7681-82-5View Details
Sodium iodide CAS 7681-82-5View Details
7681-82-5 -
 Sodium iodide CAS 7681-82-5View Details
Sodium iodide CAS 7681-82-5View Details
7681-82-5